Abstract
Manganese dioxide (MnO2) films are developed by potentiostatic electrodeposition on a stainless steel sheet as a current collector using manganese acetate tetrahydrate solution at a concentration of 0.25 mol L−1. The deposited sample layer is found to be amorphous as confirmed by the scanning electron microscope (SEM) and X-ray diffraction (XRD) analyses. The capacitive property of the prepared electrodes is characterized by charging/discharging, cyclic voltammetry, and electrochemical impedance spectroscopy techniques in four different aqueous solutions; sodium sulfate, magnesium acetate, nickel acetate, and a mixture of sodium chloride + sodium bicarbonate + borax. Charge–discharge curves reveal higher specific capacitance (Cs) of 1580 F g−1 at 0.5 mA cm−2 for the investigated MnO2 film which is characterized in Ni acetate aqueous solution. The structural, morphological, and electrochemical properties of the electrodeposited manganese oxide films are also studied. The results indicate that the specific capacitance of the deposited samples showed a strong dependency on the electrolyte aqueous solutions, resulting in developing new electrolytes which is a top priority effort in comparison to seeking new electrode materials. It is found that the matching between the pore size structure of the electrode and the ion size of the electrolyte is of great importance for the improved capacity of prepared capacitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The storage of massive amounts of energy is a major obstacle to the production of electricity. For many years, scientists have researched ways to store energy and boost efficiency. Researchers have been looking into novel materials and methods to store energy more effectively and in larger quantities in recent years. These scientific investigations are being prompted, specifically, by technology related to electric vehicles and renewable energy sources. Recently, the supercapacitor (SC) was presented as an alternating or hybrid storage device by scientists and producers [1]. Supercapacitors are electrochemical, unconventional, and high-performance energy storage devices working on the rule of electrochemical energy conversion that store and discharge energy by nanoscopic charge separation at the interface between the electrode material and the electrolyte. Those supercapacitors are characterized by high power density, high specific capacitance, good durability, high stability, and low fabrication cost. Those devices experience no memory effect, are safe, and collect the specifications of electrolytic capacitors with high power density and fuel cells or rechargeable batteries with large energy density [2, 3]. Pseudocapacitors, in which the electrical charges are mainly stored by reversible and fast redox reactions [4,5,6,7] have attracted great attention from researchers for studying and finding suitable electrode materials for improving the performance of pseudocapacitors. This is because of their higher energy density but lower power density as compared with electric double-layer capacitors (EDLCs) which often lack cyclic stability. Manganese oxide (MnOx) among the different transition metal oxides is considered a promising electroactive pseudocapacitive electrode material for supercapacitors due to its excellent theoretical capacitance ranging from 1100 to 1300 F g−1 in the aqueous electrolytes [8], environmental compatibility, low-cost, large voltage window [9, 10], and abundant availability on earth. Electrochemical deposition methods are grouped into two different types: anodic and cathodic. However, the anodic electrochemical deposition of MnO2 is commonly used [11]. In conclusion, it should be noted that the specific capacity of the capacitor, as expressed in F g−1, is mostly dependent on the capacity of the electrode material, while the voltage and the resistance of such device depend mainly on the electrolyte.
This work aims to explore new innovative electrolytes by studying the potentiostatic electrochemically deposited thin films of MnO2 on stainless steel substrate SS-304 as a current collector by anodic electrodeposition method using manganese acetate solution, and the characterization will be performed in four different aqueous solutions; sodium sulfate, magnesium acetate, nickel acetate, and a mixture with an equal ratio from sodium chloride + sodium bicarbonate + borax (S.S.B), whereas the matching between the pore size of the electrode film and ion size of the electrolyte is an important factor for obtaining a supercapacitor with an improved capacity.
2 Experimental
2.1 Materials
Used materials in this work are: manganese acetate tetrahydrate (Mn(CH3COO)2.4H2O), Sodium sulfate (Na2SO4), Magnesium acetate (Mg(CH3COO)2.4H2O), S.S.B. (an equal mixture of Sodium chloride (NaCl) + Sodium bicarbonate (NaHCO3) + Sodium borate (Na2B4O7.10H2O)), Ni Acetate (Ni(CH3COO)2.4H2O)), commercially stainless steel (SS) sheet type 304 of thickness 0.175 mm, Sulphuric acid (H2SO4), and Deionized Water (DW).
2.2 Characterization and synthesis of MnO2 electrodes
2.2.1 Structural, and morphological characterizations
The structural and morphological characteristics of prepared MnO2 films were examined by X-ray diffraction (XRD) using PANalytical co Xpert PRO Cu target, λ = 1.54 Å, scanning electron microscope (SEM) at some different magnifications (500, 100, 4, and 2 µm), and electron dispersive spectroscopy (EDX) using QUANTA FEG 250.
2.2.2 Electrochemical preparation
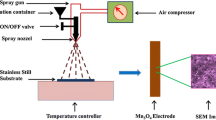
For the synthesis of MnO2 electrodes by an electrochemical deposition method, a stainless steel sheet was cut as samples of face area 1 × 2 cm2 to be considered as a working electrode for the deposition. The samples were first etched in H2SO4 98% for 30 min, and then washed with distilled water and air-dried. The MnO2 thin films with mass loading of 50 µg cm−2 each, were anodically electrodeposited using potentiostatic (PS) condition at 1 Volt using 0.25 mol L−1 manganese acetate [(CH3COO)2 Mn.4H2O] solution at room temperature, and the deposited area was 2 cm2 (both sample faces). The deposited mass was controlled by adjusting the total charge passed through the electrode during deposition time. The electrochemical deposition and characterization of the electrodes were performed using the conventional three-electrode system through EC-Lab software of SP-150 potentiostat/galvanostat device in a cell with MnO2/stainless steel substrate as a working electrode, Ag/AgCl (KCl saturated) as a reference electrode, and platinum wire as a counter electrode. The prepared samples were conducted as electrodes for energy storage in different electrolyte solutions [0.5 mol L−1 of Na2SO4, 0.5 mol L−1 of Mg Acetate, 0.25 mol L−1 of Ni Acetate, and 1.25 g of S.S.B. in 50 mL distilled water]. The charge–discharge technique is used at current densities in a range of 0.5–5 mA cm−2 in a voltage window between 0 and 1 V, cyclic voltammetry in the potential range (0–1 V) with different scan rates of 10–100 mV s−1, and electrochemical impedance spectroscopy (EIS) recorded using sine wave voltage amplitude of 10 mV superimposed on 10 mV DC voltage, and frequency range of 10 mHz–100 kHz. The exact method for measuring the specific capacitance of supercapacitor electrodes is the galvanostatic charge–discharge method, which has an exact value for practical applications.
3 Results and discussion
3.1 Structural, and morphological investigations
3.1.1 X-ray diffraction
Figure 1 shows XRD patterns of both the etched bare stainless steel and the electrodeposited MnO2 film on the etched SS substrate. The evolution of sharp peaks in X-ray for the etched bare stainless steel (SS) reflects the crystalline structure of the stainless steel substrate. When the potentiostatic deposition of MnO2 film on a stainless steel substrate was conducted, the X-ray diffraction plot was recorded again and showed low intensity due to the deposited film. The obtained XRD records of tested specimens are shown in Fig. 1. It shows no distinct peaks other than those corresponding to the SS substrate peaks, which probably indicates that the deposited MnO2 colloidal particles are in an amorphous phase [12] and all deposited MnO2 films are too thin [13]. Therefore, the obtained X-ray diffraction patterns confirm the amorphous nature of the deposited MnO2 layer. The peaks observed at 2θ = 42.5° and 50.5° confirm the formation of α-MnO2, indexed by JCPDS NO. 44-014 [14] which corresponds to the (110) crystallographic plane located beside the broadened one indicating that there is some γ-MnO2 with good crystallinity [15], and the planes of α-MnO2 are (411). Also, the diffraction peak is broad indicating a poor crystallinity and small grain size of α-MnO2 [16].
The detected X-ray peak roots which are superimposed on the pattern of MnO2 at the same location of SS crystalline peaks are due to a higher order of X-ray reflections from SS substrate. This indicates the amorphous nature of the metal oxide film which is generally required for obtaining a large surface area for supercapacitor electrodes, where the amorphous phase can enable the ions to penetrate such bulk active material [17].
3.1.2 (SEM and EDX) spectroscopy
Figure 2a shows that the surface morphology of deposited manganese oxide has regular striped features that look like to be bright at low magnification (500 µm). Figure 2d indicates that the surface morphology of deposited manganese oxide reveals the highly porous structure of the oxide at high magnification (2 µm). Also, Fig. 2b and c show the two magnifications (100, and 4 µm) between the above lowest and highest magnifications. The SEM micrographs in the four figures revealed different surface appearances and proved the amorphous structure of the prepared electrode, that the X-ray structure detected, as previously discussed in this work.
The EDX results of the deposited MnO2 film are shown in Table 1, have been used to confirm the formation of MnO2 on SS substrate, the compositions and weights (%) of C, O, Cr, Mn, Fe, and Ni are displayed which confirms the presence of Mn and the successful deposition of MnO2 on the SS substrate. The atomic percentage of the electrode shows the presence of 5.25% Mn, and 23.99% O which belong to MnO2 products, while other elements such as small amounts of, 6.61% Cr, 27.35% Fe, 33.79% C, and 3.01% Ni originated from SS [18]. The elemental EDX mapping of Mn is shown in Table 1 and Fig. 2a, b, c, d and e of SEM, confirming the homogenous distribution of Mn, Cr, Ni, Fe, O, and C elements on the SS substrate surface.
3.2 Electrochemical measurements
3.2.1 Charge–discharge (CD) behavior
The best technique for measuring the specific capacitance of supercapacitor electrodes is the charge–discharge method, which has an exact value for practical applications. Charge–discharge process for deposited MnO2 films was conducted using the chronopotentiometry technique with a potential window between 0 and 1 Volt in [0.5 mol L− Na2SO4, 0.5 mol L−1 Mg Acetate, 1.25 g of S.S.B. in the 50 mL distilled water, and 0.25 mol L− Ni Acetate] electrolytes at different current densities from 0.5–5 mA cm−2 at room temperature.
Figure 3a shows the charge–discharge (CD) curves for deposited MnO2 films at a current density of 0.5 mA cm−2, and Fig. 3b gives the specific capacitance values of deposited MnO2 against different current densities ranging from 10 to 100 A g−1 for the four electrolyte solutions.
a CD curves of deposited MnO2 electrodes at an actual current density of 1 mA in different solution electrolytes: Na2SO4, Mg Acetate, S.S.B., and Ni Acetate. b Variation of specific capacitance with current densities (from 10 to 100 A g−1) using different electrolyte solutions (Na2SO4, Mg Acetate, S.S.B., and Ni Acetate) for MnO2/SS electrode film
As shown in Fig. 3a, the quasi-triangular shaped and symmetrical charge/discharge curves for the deposited MnO2 films of mass 50 µg cm−2 indicate the capacitive characteristics of the tested MnO2 electrodes demonstrating good capacitive property and reversibility of the electrochemical reactions in the MnO2 films. CD curves of Fig. 3a show evidence, that Ni Acetate solution takes the longest time to discharge, and this is required for supercapacitors for enhancing their capacitance. CD curves of Fig. 3a for all the investigated electrodes also show a DC internal resistance exists at the switching point of potential. The values of specific capacitance were obtained from charge–discharge curves using the following equation [1]:
where I is the discharge current, m is the mass of the electrode film, and dV/dt is the slope of the discharge half-cycle.
The variation of specific capacitances and voltage drop for all samples, shown in Table 2, indicates that Na2SO4 electrolyte has the lowest specific capacitance of 467.5 F g−1 and the lowest voltage drop of 0.041 V, followed by Mg Acetate, and S.S.B. electrolytes that have close specific capacitance values (841.5, and 748.5 F g−1) with the highest voltage drop values (0.113, and 0.149 V), while Ni Acetate has the highest specific capacitance of 1580 F g−1 with lower voltage drop value of 0.09 V; close to the lowest value of voltage drop of Na2SO4 electrolyte (0.041 V).
To the best of our knowledge, S.S.B., Mg Acetate, and Ni Acetate are new electrolyte solutions and were not tested before. From our investigation, it was found that Ni Acetate solution is the best characterization solution that has the highest specific capacitance value with a small voltage drop as compared with the other investigated electrolyte solutions.
Figure 3b shows that the specific capacitance decreases as the current density increases which occurs in common behavior. It was found Na2SO4 electrolyte (the lowest SC), slightly decreased in SC starting from 467.5 F g−1 at the lowest current density (10 A g−1), to 329.1 F g−1 at the highest current density (100 A g−1). For Mg Acetate and S.S.B. electrolytes (higher SC than Na2SO4), a higher decrease in SC was obtained starting from (841.5 and 748.5 F g−1) at the lowest current density to (157.4 and 347.1 F g−1) at the highest current density, respectively. For Ni Acetate salt (the highest SC), a clear higher decrease in SC was achieved starting from 1580 F.g−1 at the lowest current density to 284.7 F.g−1 at the highest current density. This common decrease is due to the limited accessibility of ions (or charge carriers) to the electrode pores at high-applied electric currents.
3.2.2 Cyclic voltammetry (CV) behavior
Among electrochemical techniques, CV is widely used to determine the behavior of charges at the electrode–electrolyte interface in a specific potential range [19, 20]. In this technique, the potential of the working electrode is scanned at a constant rate (ΔV/Δt) in a potential window for many cycles. The resulting current values are measured and plotted as a function of the applied voltage. This technique is a useful and simple technique to determine capacitance, particularly for electrochemical double-layer capacitors [21, 22].
The cyclic voltammetry curves of the deposited MnO2 films of mass loading 50 µg cm−2 on etched SS electrodes were obtained using different electrolyte solutions at different scan rates, from 10 mV s−1 to 100 mV s−1, in the potential range, from 0 to 1 V for each electrolyte solution at room temperature.
Figure 4a shows the CV plots at a scan rate of 10 mV s−1 (cycle no. 2) for the tested samples using different electrolyte solutions at specified concentrations. Those curves indicate that rectangular curves without redox peaks are obtained in the tested potential window, indicating the existence of a high capacitive behavior with a good response of ion (or charge carrier) transfer. Figure 4b shows the specific capacitance values of deposited MnO2 films against scan rates from 10 to 100 mV s−1 for the four investigated electrolytes. As commonly known, the specific capacitance decreases as the scan rate increases. Using Na2SO4 electrolyte (the lowest SC), there is a slight decrease in SC starting from 446.1 F g−1 at the lowest scan rate (10 mV s−1), to 323.8 F g−1 at the highest scan rate (100 mV s−1). Using Mg Acetate, and S.S.B. electrolytes (higher SC than Na2SO4), there is a higher decrease in SC starting from (638.4, and 758.8 F g−1) at the lowest scan rate to (295.2 and 356.7 F g−1) at the highest scan rate, respectively. Using Ni Acetate electrolyte (the highest SC), there is a clear sharp decrease in SC starting from 1120.2 F g−1 at the lowest scan rate, to 284.7 F g−1 at the highest scan rate. This relation verifies the basic capacity potential rate relation and also shows that the capacity of the deposited layer of MnO2 investigated in Ni Acetate electrolyte solution is the highest one of the other used solutions and corresponds to CD results.
The specific capacitance in (F g−1) of the deposited film is given by the amount of capacitive charge, Q in coulombs (equals half of the integrated area of each of the corresponding CV curves presented in Fig. 4a divided by the product of the film mass, m in grams multiplied by the width of the applied potential window, ∆V in Volts.
Table 3 shows the values of specific capacitances for all investigated electrolytes at a scan rate of 10 mV s−1. The values match the data from CD curves and proved that Na2SO4 electrolyte has the lowest specific capacitance than all other electrolytes, 446.1 F g−1, followed by Mg Acetate, and S.S.B. have specific capacitances higher than Na2SO4 (638.4, and 758.8 F g−1), and Ni Acetate salt has the highest specific capacitance of 1120.2 F g−1.
Figure 5a, b, c, and d shows the typical cyclic voltammogram curves in a scan rate range of 10–100 mV s−1 for the deposited MnO2 electrodes. It can be seen that the curves of CV keep a symmetrically rectangular form even at a scan rate of 100 mV s−1, it remains symmetric and rectangular, showing excellent high-rate performance.
3.2.3 Electrochemical impedance spectroscopy (EIS)
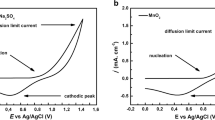
Electrochemical impedance spectroscopy is used to study the charging/discharging of the electrode materials using an alternating current voltage to determine their ionic and electronic resistances. As known, the capability of power output from supercapacitors depends on the rates of ionic mass transport [23] and the equivalent series resistance [24] (ESR). The ESR represents the ionic resistance of the electrolyte, the resistance of the active material, the resistance of the current collector, and the contact resistance at the electrode/electrolyte interface. It is the sum of two major parts, an electronic resistance and an ionic one. The interfacial reaction kinetics [17, 23] are represented by the charge transfer resistance (Rct) obtained by the diameter of the semicircle in the high-frequency region. Figures 6a, b show the measured Nyquist plots for the deposited MnO2 electrodes recorded in the frequency range of 10 mHz–100 kHz, under applying an alternating current of 10 mV voltage amplitude and studied in different electrolyte solutions (Na2SO4, Mg Acetate, S.S.B., and Ni Acetate), respectively.
Table 4 shows values of Equivalent series resistance, and charge transfer resistance values for the potentiostatically deposited MnO2/SS characterized in different electrolyte solutions (Na2SO4, Mg Acetate, S.S.B., and Ni Acetate).
As indicated in Table 4, the ESR and Rct values for the prepared MnO2 electrode increase by changing the electrolyte type in the following arrangement (Na2SO4, S.S.B., Mg Acetate, and Ni Acetate). The ESR increase with electrolytes toward Ni Acetate reflects the higher ionic resistance of the electrolyte leading to a corresponding increase in the charge transfer resistance.
3.2.4 Power density (P.D.), and energy density (E.D.)
Figure 7 shows the calculated values of power density obtained from the Nyquist plot, and the energy density at a current density of 0.5 mA cm−2 for the deposited MnO2 electrodes studied in the four electrolyte solutions (Na2SO4, Mg Acetate, S.S.B., and Ni Acetate) as given from equations [3, 4]:
It is shown in Fig. 7 that the sample investigated in the Na2SO4 solution has the highest power density (406.5 kW kg−1), with the lowest value of ESR as mentioned before. This is in agreement with the fact that power density is inversely proportional to the ESR as given in Eq. 3 and also has the lowest E.D. (64.9 Wh kg−1). The sample studied in Ni Acetate solution has the highest energy density (219.4 Wh kg−1) as it has the highest SC as given in Table 1 from CD curves mentioned before. This is in agreement with the fact that energy density is directly proportional to SC as derived from Eq. 4 and has the lowest power density (130.2 kW kg−1).
3.2.5 Stability using CD curves
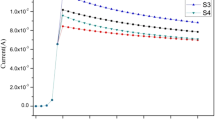
The life-cycle test was selected for the deposited MnO2 film in the two solutions (S.S.B., and Ni acetate) performed at a discharge current density of 1.5 mA cm−2 for 9000 cycles as presented in Fig. 8.
As shown, using S.S.B. as an electrolyte, there is a noticed decrease in the value of specific capacitance from cycle 1 to cycle 500 (from 489.7 to 397 F g−1), then from cycle 500 to cycle 3000 there is a slight increase from (397 to 409.6 F g−1), after that from cycle 3000 there is some slight increase and decrease until reaching cycle 9000 (at 399.2 F g−1), i.e., the specific capacitance value remained almost constant during cycles from 500 to 9000.
While using Ni acetate, there is an increase in the value of specific capacitance from cycle 1 to cycle 500 (from 544.9 to 571.5 F g−1), then from cycle 500 to cycle 3500 there is a noticed decrease (at 368 F g−1), and from cycle 3500 to cycle 9000 there is an increase in specific capacitance until reaching a value of 398.9 F g−1.
The results of Fig. 8 suggest that the studied electrode in the S.S.B. solution has good cycling stability as compared with the electrode in Ni acetate, however, the start value of specific capacitance in Ni Acetate is higher than that in S.S.B.
4 Conclusion
Novel electrolyte solutions (sodium sulfate, magnesium acetate, nickel acetate, and a mixture of sodium chloride + sodium bicarbonate + borax) were developed for MnO2 electrodes with mass loading of 50 µg cm−2, electrodeposited on an etched stainless steel current collector from 0.25 Mole L−1 manganese acetate solution with low-cost procedure giving a high capacitive performance represented in the high energy density. The structure, morphology, and element composition of the prepared deposited electrode were characterized by X-ray, SEM, and EDX. The obtained results showed the amorphous nature of the prepared sample. The prepared films were tested as an electrode for the supercapacitor application, and characterized by the charging/discharging (CD) technique, cyclic voltammetry technique, and electrochemical impedance spectroscopy. The deposited MnO2 film characterized in Ni Acetate solution (as compared with S.S.B., Mg Acetate, and Na2SO4) has the highest Cs of 1580 F g−1 at a current density of 0.5 mA cm−2, with the highest energy density of 219.4 Wh kg−1. Thus, a new category of novel electrolytes is used in this work and are considered promising electrolytes for the anodically deposited MnO2 films on the stainless steel substrates for potential supercapacitor applications. Our objective is to develop a manganese Oxide (MnO2) electrode to improve supercapacitor electrochemical performance, with both high energy and power densities maintaining the long cycle life to be used in low-cost energy storage devices applications in deposition electrolytes. Our ultimate goals are to synthesize MnO2 electrodes under different conditions using different novel electrolyte solutions, and then fabricate the advanced energy storage device for scientific and industrial applications. Finally, it was found the enhanced capacity of produced capacitors greatly depends on the matching between the pore size structure of the electrode and the ion size of the investigated electrolyte.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
Change history
04 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10854-024-13027-z
References
M.E. Şahin, F. Blaabjerg, A. Sangwongwanich, Energies 15, 674 (2022)
L.L. Zhang, X.S. Zhao, Chem. Soc. Rev. 38, 2520 (2009). https://doi.org/10.1039/b813846j
A. Balducci, R. Dugas, P.L. Taberna et al., J. Power. Sour. 165, 922 (2007). https://doi.org/10.1016/j.jpowsour.2006.12.048
B.E. Conway, in Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications. ed. by B.E. Conway (Springer, Boston, 1999)
P. Simon, Y. Gogotsi, Nat. Mater. 7, 845 (2008)
A.S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon, W. van Schalkwijk, Nat. Mater. 4, 366 (2005). https://doi.org/10.1038/nmat1368
M. Winter, R.J. Brodd, Chem. Rev. 104, 4245 (2004). https://doi.org/10.1021/cr020730k
V. Augustyn, P. Simon, B. Dunn, Energy Environ. Sci. 7, 1597 (2014). https://doi.org/10.1039/c3ee44164d
R.C. MacCallum, S. Hong, Multivar. Behav. Res. 32, 193 (1997)
W. Wei, X. Cui, W. Chen, D.G. Ivey, Chem. Soc. Rev. 40, 1697 (2011). https://doi.org/10.1039/c0cs00127a
G.A.M. Ali, M.M. Yusoff, Y.H. Ng, H.N. Lim, K.F. Chong, Curr. Appl. Phys. 15, 1143 (2015). https://doi.org/10.1016/j.cap.2015.06.022
D.P. Dubal, D.S. Dhawale, T.P. Gujar, C.D. Lokhande, Appl. Surf. Sci. 257, 3378 (2011). https://doi.org/10.1016/j.apsusc.2010.11.028
K. Takahashi, Y. Wang, G. Cao, J. Phys. Chem. B 109, 48 (2005). https://doi.org/10.1021/jp044772j
E. Beaudrouet, A. Le Gal La, D.G. Salle, Electrochim. Acta 54, 1240 (2009). https://doi.org/10.1016/j.electacta.2008.08.072
Y. Chabre, J. Pannetier, Prog. Solid State Chem. 23, 1 (1995)
C.-L. Ho, M.-S. Wu, J. Phy. Chem. C. 115, 22068 (2011). https://doi.org/10.1021/jp2081337
S. Hassan, M. Suzuki, A.A. El-Moneim, Am. J. Mater. Sci. 2(2), 11–14 (2012)
D. Su, J. Ma, M. Huang et al., IOP Conf. Ser.: Mater. Sci. Eng. 207, 012087 (2017). https://doi.org/10.1088/1757-899x/207/1/012087
J.W. Patrick, Fuel 83, 623 (2004). https://doi.org/10.1016/j.fuel.2003.09.012
J.A. Gardiner, J.W. Collat, J. Am. Chem. Soc. 87, 1692 (1965). https://doi.org/10.1021/ja01086a013
T. Tevi, H. Yaghoubi, J. Wang, A. Takshi, J. Power. Sources 241, 589 (2013). https://doi.org/10.1016/j.jpowsour.2013.04.150
T. Tevi, S.W. SaintBirch, S.W. Thomas, A. Takshi, Synth. Met. 191, 59–65 (2014). https://doi.org/10.1016/j.synthmet.2014.02.005
A. Izadi-Najafabadi, D.T.H. Tan, J.D. Madden, Synth. Met. 152, 129 (2005). https://doi.org/10.1016/j.synthmet.2005.07.094
A. Celzard, F. Collas, J.F. Marêché, G. Furdin, I. Rey, J. Power Sour. 108, 153 (2002). https://doi.org/10.1016/S0378-7753(02)00030-7
Acknowledgements
This work was supported financially by the Science and Technology Development Fund (STDF), Egypt (grant no 39476). The authors acknowledge Menoufia University- Faculty of Science- Physics department.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by Science and Technology Development Fund (STDF) Grant No. (39476).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, S., Khafagy, A.H. & Usama, D. Novel promising aqueous electrolytes for manganese dioxide/stainless steel pseudocapacitor electrode. J Mater Sci: Mater Electron 35, 1123 (2024). https://doi.org/10.1007/s10854-024-12803-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12803-1