Abstract
Effect of incorporation of sensitizers namely palladium (Pd) and platinum (Pt) on the gas-sensing behaviour of zinc oxide (ZnO) nanowires has been studied. The specificity achieved is further studied and demonstrated for its efficacy towards the simultaneous detection of multiple gases employing the developed sensors in an electronic nose configuration. Incorporation of salt solutions containing the desired sensitizer concentration in the starting reaction mixture of hydrothermal growth has been effectively used to achieve heterostructure ZnO nanowires. Pd and Pt gets incorporated as PdO and metallic Pt, in the host matrix resulting in the formation of random heterojunctions namely p–n junction and Schottky junctions. Consequently, an increase in the work function as studied using Kelvin probe studies is observed. Utilizing statistical implements namely principal component analysis (PCA) and hierarchical cluster analysis (HCA) the discrimination of three gases namely H2, H2S and NO2 has been successfully accomplished. 3D PCA discriminates the three gases successfully with first three components exhibiting a percentage of variance of 42.32, 33.26 and 24.20%, respectively. A reasonable discrimination of H2, H2S and NO2, grouped into three clusters as evident from HCA dendrograms, was achieved using utilizing Ward’s method and Euclidian distance metric approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ZnO is a multifarious functional material, which has exhibited itself in the diversity of growth forms such as nanocombs, nanobelts, nanorings and nanowires (NWs) [1,2,3]. The associated high surface area-to-volume ratio coupled with the inherent oxygen non-stoichiometry has been effectively used to realize sensors with tailor response towards target gases. In pure form, ZnO often suffers from the drawback of cross selectivity i.e. nearly identical response characteristics is observed for all the gases. Accordingly, to improve the sensor response and selectivity towards the required gases, different approaches have been investigated. Surface modification with sensitizers like Au, CuO, NiO, PdO and Pt [4,5,6,7] is one of the most commonly employed method. Incorporation of sensitizers has been found to improve the response characteristics, which is attributed to the increase in the number of gas-specific selective sites for interaction with the target gas [8,9,10]. This has been predominantly explained using the chemical and electronic sensitization methods [11, 12]. In chemical sensitization, the sensitizers help to increase the oxygen coverage over the sensor surface thereby increasing the available sites for interaction. And in the electronic sensitization, the sensitizer itself takes part in the sensing mechanism. It provides the additional mid-gap states by forming a junction with the host material like p–n junction or Schottky junction. The resulting barriers have been found to have a strong dependence on the sensor environment and accordingly, the presence of target gases is expected to drastically alter the sensor properties. For example, we have demonstrated earlier an improvement in sensitivity and selectivity of ZnO NWs towards H2S and NO2 gases employing Au, CuO, TiO2 and Al layer on top of the NWs [13,14,15,16]. Herein, the modulation in the response characteristics was governed by the creation and the breakdown of the p–n and Schottky barriers between sensitizer and ZnO [17, 18]. One of the crucial parameters to achieve the good sensor response characteristics is the control over sensitizer expanse and uniform dispersal on the sensor surface. If the amount is too low, it would not yield the improved sensor performances and if it is too high, the sensitizer itself dominants and consequently, the response characteristics achieved are those of pure samples which are often low. Hence, it is desired to find the way to achieve optimum concentration and distribution of the sensitizer over the sensor surface. The sensor is judged on its important sensor parameters, which includes sensitivity, specificity, faster response kinetics and life. Sensitivity is the ability of the sensor to produce maximum change in its properties for a small change in the gas concentration. It is defined as the sensor response per unit gas concentration. Selectivity is the ability of the sensor to produce maximum change when exposed to the target gas in the presence of other interfering gases. The sensor exhibiting highly repeatable and reproducible measurements with minimum drift in its response characteristics over a long-term measurement periods (> 3 months) is considered to be highly stable.
To identify the sensor with potential for possible commercial viability, the sensor needs to satisfy the “4-S sensor selection or Ramgir criteria” [19, 20]. Herein, each S stands for high sensitivity, selectivity, stability and suitability. First three ‘S’s are related to the performance of the sensor, while the fourth ‘S’ takes into consideration of economic viability. It is desirable of the sensor to fulfil the criteria so as to stand a chance of commercial deployment and acceptance. In particular, the sensor with high sensitivity, selectivity towards a target gas, exhibiting a long-term stable performance under operating conditions and importantly having cost effective complete sensor fabrication process will find its place in the market. Accordingly, attempts are being directed towards finding the novel sensor configuration and methodologies to find sensors that could satisfy the above-mentioned criteria.
Now, in the similar context to surface modification, incorporation of sensitizers into the host material can also be looked upon as a potential way to achieve the improvement in sensing characteristics. Herein, again the amount and distribution of sensitizers in the host matrix is expected to play crucial role in determining the sensing characteristics. It is important to note that ZnO exhibits itself into hexagonal wurtzite structure that is characterized by the presence of alternate layers of Zn and O. ZnO NW structure in particular is characterized by the presence of polar Zn and O layers as top and basal planes with six non-polar side planes [21, 22]. It is noteworthy to mention that during growth, incorporation of impurities usually takes place along the side planes, thereby could be looked upon as a potential means to tailor the sensor response. Hence, Pd and Pt having ionic radii of 1.35 and 1.40 Å close to that of Zn (1.30 Å) are expected to easily get incorporated into the ZnO matrix and could help to achieve improved sensor response characteristics. Both Pd and Pt are known to act as a catalyst for H2 oxidation and expected to improve the response characteristics. Besides, Pd could form PdO upon oxidation and help to increase the effective number of oxygen species on the sensor surface and being a p-type material might form a p–n junction. Contrary, Pt will get incorporated as Pt itself and may form Schottky barrier with ZnO. The formation and modulation or collapse of these properties may help to improve the response characteristics.
Accordingly, in the present work, incorporation of Pd and Pt into ZnO matrix has been investigated as a means to achieve improved response characteristics towards H2, H2S and NO2. For this, incorporation of salt solution containing the desired sensitizer concentration in the starting reaction mixture of hydrothermal growth has been effectively used. Pd and Pt have been found to be present as PdO and metallic Pt in the ZnO matrix and consequently resulted in the formation of random heterojunctions namely p–n and Schottky junctions, respectively. This has resulted in the sensor film exhibiting specific response towards H2, H2S and NO2, which are subsequently being studied for their usage in an electronic nose configuration for simultaneous detection of multiple gases. Using the developed e-nose and the statistical implements namely principal component analysis (PCA) and hierarchical cluster analysis (HCA) the discrimination of three gases namely H2, H2S and NO2 has been successfully accomplished.
2 Experimental section
2.1 Synthesis of pure and Pd- and Pt-incorporated ZnO NWs
Synthesis of ZnO NWs was carried out using hydrothermal method [2]. An initial chemical route was used to synthesize ZnO nanoparticles (NPs), which were stable up to two-week period. For nanowire growth, the solution of NPs was spin-coated onto glass substrates of size 1 × 1 cm2. Before spin coating, the substrates were cleaned with a UV ozone cleaner. Spin coating was performed in two stages, namely 500 rpm for 20 s and 1000 rpm for 30 s. For better adhesion, the spin-coated substrates were annealed at 150 °C for 1 h. The hydrothermal growth of pure ZnO NWs and with Pt and Pd sensitizers were carried out using the following method. For Pt and Pd modification, a micromolar solution of hexachloroplatinic acid and palladium chloride, respectively, was added to an aqueous equimolar (25 mM) solution of zinc nitrate hexahydrate and hexamethylenetetramine. In addition, the substrates coated with ZnO NPs were suspended upside down at 95 °C for 7 h. The resulting NW films were then washed first in deionized water several times and later dried underneath Ar lamp. Subsequently, the samples were annealed at 400℃ for 1 h in the presence of 25 sccm oxygen. The samples were designated as pure ZnO NW, ZnO NW-Pt and ZnO NW-Pd, respectively.
2.2 Morphological and structural characterization
For the determination of the morphology and elemental quantization, scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) measurements were performed on SERON Evo18 instrument. For X-ray powder diffraction (XRD), the INEL XRG 3000 instrument with CuKα line (λ = 1.5406 Å) was used. The presence of Pd and Pt and their chemical states were investigated by X-ray photoelectron spectroscopy (XPS) using a MgKα x-ray source (1253.6 eV) and a DESA-150 electron analyzer (Staib Instruments, Germany). Work function measurements before and after exposure to test gases have been performed using the SKP Kelvin Probe 4.5 (M/s. KP Technology Ltd., Scotland UK) [23,24,25].
2.3 Gas-sensing studies
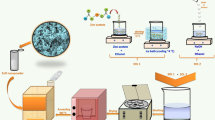
Figure 1 indicates the schematic representation of chemiresistive e-nose gas measurement system. The device is enabled with data acquisition and static test chambers (250 ml) consisting of seven individual measuring channels. A channel is connected to a known 1 kΩ resistor for calibration of sensor resistance values. The operating temperature of each sensor was set using Pt-100-based heater and temperature control cards. For sensor measurements, gold electrodes were deposited by thermal evaporation method at 5 × 10–5 mbar pressure with the separation between the Au electrode contacts being 1 mm. The response curves of the sensor were plotted, and the typical parameters namely sensor response, response time and recovery time were measured. Sensor response is defined as the ratio of the resistance of the sensor film in the target gas to that in air. The response time is defined as the time required to reach 90% of the final value when the sensor is exposed to target gas. Contrary, recovery time is the time required to attend 10% of the saturation value upon exposure to ambient. By adding response and recovery times, the total time was calculated.
Using the estimated values, the data repository was created as a function of gas, gas concentration and operating temperature. Further the data were interpreted using the multivariate data analysis technique, which helps to interpret the complicated electronic nose data. The present investigation is limited to two cluster analysis method namely hierarchical cluster analysis (HCA) and principal component analysis (PCA), which can be used for qualitative and quantitative discrimination of gases.
3 Results and discussion
3.1 Structural and elemental analysis
The hydrothermal growth of NWs was carried out using different concentrations of for Pt and Pd, (0.5 to 4 μM) in the reaction mixture. The NWs morphology was found to collapse for concentration ≤ 2 μM. Figure 2a–b shows the SEM image for the pure ZnO and the samples grown using 1 μM concentration in the reaction mixture. For pure ZnO NWs, the diameters were observed to be from 50 to 200 nm, while length was found to be 1–2 µM15. For Pd- and Pt-incorporated samples, NWs having diameter in the range from 160 to 220 nm and from 300 to 450 nm, respectively, were observed. Thus, it is clear that the incorporation of sensitizers causes an increase in the diameter of the resulting NWs [22]. Figure 2c–d shows the EDS spectra of the samples, wherein the presence of zinc, Pt, Pd and oxygen is confirmed. Impurities, in the present case Pd or Pt are expected to get adsorbed along the non-polar directions and hence an increase in the diameter is observed. Higher concentration, however, leads to the collapse of the NW morphology.
Figure 3a shows the XRD pattern of a pristine, randomly oriented ZnO NW network. The presence of (1 0 0) (0 0 2), (1 0 1) and (1 0 2) peaks confirms the formation of ZnO. No characteristic peaks corresponding to the zinc nitrate and HMTA were observed. The peaks corresponding to Pt and Pd were not observed because their concentrations were well below the detection limit of XRD (i.e. 5 wt.%). To further confirm the presence and determine the chemical and oxidation state of the elements, XPS measurements were performed. Figure 3 (b) shows the survey spectrum for all the samples. The binding energies (BE) of the peaks were calibrated using the Au-4f 7/2 peak (84.0 eV) as a reference, which showed the random C-1 s peak at 284.8 eV.
The survey spectrum confirms the presence of Zn, Pd, Pt and O peaks. Figure 4 shows the deconvoluted peaks corresponding to Zn, O, Pt and Pd samples. The deconvoluted peaks corresponding to small concentration of Pd (1 µM) were found to be suppressed due to strong Zn-Auger peaks. Hence, the XPS analysis for pristine Pd (1 µM) sample was carried out independently. In the XPS spectra, the plot with the hollow circles shows the original data, while the solid lines represent both the fitted curve and the deconvoluted individual peaks of the different species. For pure ZnO NWs, the Zn-2p doublet peaks corresponding to 2p3/2 and 2p1/2, at 1022.5 and 1045.4 eV, respectively[26,27,28], were observed to shift to lower BE values of 1020.8 and 1043.9 eV and 1020.5 and 1043.5 eV, for Pd- and Pt-incorporated samples, respectively. Figure 4b shows the deconvoluted O-1 s spectra for pure, ZnO NWs-Pd and ZnO NWs-Pt, samples. For pure ZnO, the O-1 s spectrum can be deconvoluted into two peaks at 530.1 and 531.6 eV, corresponding to lattice oxygen and chemisorbed oxygen species, respectively [29]. Similarly, for Pd- and Pt-incorporated NWs, the deconvoluted peaks were found at 531.0 and 532.5 eV and 530.4 and 532.1 eV, respectively.
Figure 5 shows the spectra corresponding to Pd and Pt signal [30]. As shown in Fig. 5 (a), the peaks at 337.8 and 343.3 eV correspond to 3d5/2 and 3d3/2 signal of the PdO oxidation states, respectively [31]. For Pt, the peaks observed at 73.7 and 75.5 eV (Fig. 5b) correspond to the doublet of Pt0 [32, 33] and attributed to 4f7/2 and 4f5/2, respectively [34]. Thus, the present study indicates that Pd is present as PdO, while Pt is present in the metallic state. Accordingly, the sensors are named as ZnO NWs-PdO and ZnO Nws-Pt, respectively.
3.2 Gas-sensing studies
The sensors working on the chemiresistive principle exhibit a change in resistance upon interaction with the target gases. The developed sensors exhibit partial sensitivity towards all the gases namely H2, H2S and NO2 under investigation thereby making them suitable for use in e-nose configuration as a multiple sensor array (MSA). The base resistance of the samples was determined to be 0.22, 3.5 and 0.5 MΩ for pure, ZnO NWs-Pt and ZnO NWs-PdO, respectively. This implies that the resistance increases with sensitizer incorporation and could be attributed to the increase in the defect density and formation of random barriers due to incorporation of Pd (heterojunction barrier, p–n junction) and Pt (Schottky), respectively. The sensor working temperature plays a crucial role in the adsorption and desorption of the target gases from the sensor surface. Figure 6 shows the sensor response varying with temperature for all the sensors. For H2S, the optimum temperature i.e. the temperature at which maximum sensor response is achieved, was found to be 250, 210 and 190 °C for pure, ZnO NWs-Pt and ZnO NWs-PdO, respectively. This indicates that the sensitizer helps to bring down the operating temperature, which is very much crucial from device point of view.
The response of MSA towards H2S, NO2 and H2 was subsequently investigated for different gas concentrations as shown in Fig. 7, 8 and 9, respectively. The gases have been tested in the range from 5 to 80 ppm for H2S, and 1 to 80 ppm for NO2, and 5 to 150 ppm for H2, respectively. It was observed that ZnO NWs-Pt and ZnO NWs-PdO sensors showed an improved response characteristic than pure ZnO NWs. For example, Fig. 7 shows the highest sensor response obtained for three sensors for different gases as also tabulated in Table 1. Here, the sensors for pure, ZnO NWs-Pt and ZnO NWs-PdO were designated as sensor 1, 2 and 3, respectively.
It is evident from Table 1 that sensors 2 and 3 exhibited improved SR as well as detection limit towards gases under investigation. The SR as a function of different gas concentrations of H2S, NO2 and H2 is shown in Fig. 8. Figure 9 shows the total time taken for each sensor in the MSA towards different concentrations of gases. Each sensor exhibits a unique pattern in SR and total time taken for different gases and different concentrations. No overlapping in the data was observed.
3.2.1 Gas-sensing mechanism
Both Pd and Pt can act as catalysts and facilitate the interaction of target gases through chemical sensitization and/or spill over mechanism [35]. The thermal treatment at 400 °C results in the formation of PdO, as also conspicuous from the XPS analysis (Fig. 5a). PdO being p-type in nature, forms p–n junctions with n-type ZnO NWs. And, accordingly leads to the formation of depletion layer at the interface, subsequently leading to the increase in the resistance of the sensor film. The depletion layer width can easily be changed under exposure to different target gases. On the other hand, Pt being a noble and highly stable metal do not undergo any interaction with oxygen and is present in its metallic state, as also confirmed using XPS analysis (Fig. 5b). This results in the formation of random nano-Schottky junctions with ZnO [36, 37]. Thus, incorporation of both Pd and Pt severely alters the sensor surface properties and expected to contribute towards amelioration of sensing response. SEM studies clearly indicated that incorporation of Pt and Pd does not cause any significant changes in the NWs morphology. Figure 10 shows the schematic representation of energy bands during gas-sensing measurements. Firstly, adsorption of oxygen molecules on the sensor surface results in the formation of uniform depletion layer. Now, oxygen molecule is known to get adsorbed on the sensor surface either as O2−, O− or O2−, which is governed by the sensor operating temperature. For example, for the operating temperature between 150 and 400 °C, O− ion preferably gets adsorbed, while for temperature > 400 °C, O2− dominates[38, 39]. The resulting depletion layer could easily be modulated upon exposure to oxidizing and/or reducing gases. When the sample is exposed to H2S (Fig. 10b), it interacts with the adsorbed oxygen molecules forming free electrons along with H2O and SO2 as per the following equation:
The electrons are transferred to the CB of the host material, in this case ZnO, and leads to the decrease in resistance. Both Pd and Pt incorporation increase the active sites on the ZnO NW surface and lower the surface energy, thereby favouring more gas adsorption [40]. Additionally, Pt and Pd act as catalyst for enhancing the oxygen adsorption via spill over mechanism [24]. Figure 10c represents the sensing mechanism when the samples are exposed to NO2. By nature, NO2 is an oxidizing gas and can form NO3− upon interacting with adsorbed O− ion and taking one electron from the host material as per Eq. 2.
In the present work, ZnO NWs-Pt sample shows higher resistance increase. Pt being a good catalyst, it helps in dissociation of NO2, which is initiated first with the formation of NO3 − ion as per the the following equation [41]:
Figure 10d represents the sensing mechanism when the samples are exposed to H2. Pt is also a good catalyst for H2 and it may facilitate dissociation as per Eq. 3. In particular, Pt presence helps in the dissociation of molecular H2 into atomic hydrogen species that spills over into the ZnO NW surface. These adsorbed hydrogen species interact with the adsorbed oxygen species forming water and causing release of electrons as per Eqs. 3 and 4:
For better clarity, Kelvin probe measurements were also carried out before and after subjecting the sensors to gases at an elevated temperature of 200 °C followed by quenching. The sudden decrease in temperature arrests the interactions occurring on the sensor surface, which are then measured as a work function (ϕ) variation. Figure 10a–c shows the work function 3D scan map recorded for all the samples. The work function values in air were found to be 4.97, 5.58 and 5.19 eV for pure, ZnO NWs-Pt and ZnO NWs-PdO, respectively. Incorporation of Pt and PdO has resulted in an increase in work function by ~ 0.61 and 0.22 eV, respectively. The values for work function before and after the exposure to three gases namely 25 ppm: NO2, 40 ppm: H2 and 25 ppm: H2S are tabulated in Table 2. All the samples exhibited an n-type of nature as evident from the increase and decrease in ɸ when subjected to oxidizing and/or reducing gases, respectively. For example, Fig. 11b shows the variation in work function for ZnO NWs-Pt. Exposure to 25 ppm of NO2, 40 ppm of H2 and 25 ppm of H2S has resulted in increase in ɸ to 5.63 eV (~ Δɸ = 0.05 eV), and decrease to 5.48 and 5.42 eV, respectively, corresponding to the oxidizing and reducing nature of gases.
Further, the gas adsorption kinetics was studied in detail using a mathematical model namely Elovich model. Using Elovich equation, the rate of adsorption is expressed as follows:
Here, Ɵ is the sorption capacity at time t, α is the initial adsorption rate and β is the desorption constant [42, 43]. Assuming product of adsorption rate and desorption constant being ṟ > > 1, integration of Eq. 5 gives [44]
This is a straight line equation and implies plot of θt versus ln(t) to be straight line with slope of (1/β). The sorption capacity of the sensor material denotes the rate of change of resistance upon gas adsorption. In the present work, the applicability of the Elovich model is analysed from the straight line fit to the ΔR Vs ln(t) curves for different concentrations of NO2, H2 and H2S gases. The ZnO NWs-Pt and ZnO NWs-PdO sensors have shown good sensor response values towards the test gases, and accordingly, were considered for the kinetic study. Figure 11 shows the best plot that is observed for different gas concentrations of target gases using Elovich model. To avoid the complexity of the compared data, only selective sensors are highlighted. The best linear fit is an indication of validity of Elovich model.
Further, response kinetic studies were investigated using the pseudo-second-order reaction equation given as follows:
where k is the reaction rate constant. The plot of ‘t/Rt’ Vs ‘t’ should follow the linear fit trend and hence the intercept = gives the value of ‘k’, calculating Re = 1/slope. Figure 12 shows the pseudo-second order for different concentrations of target gases.
From Fig. 13, it is observed that, the ZnO NWs-Pt and ZnO NWs-PdO, samples obey the Elovich model and also follows the second-order reaction kinetics which is also confirmed by the pseudo-second-order model. A linear fit observed in all the cases thereby confirms and proves the validity and applicability of the respective models. And it was observed that the slope of the linear fit increases with increase in the concentration of reducing gases H2S and H2, while opposite trend for oxidizing gas NO2.
3.3 Electronic nose studies
For the e-nose application, it is very important to correctly identify the gases that come into the premises of the gas sensors. Using a MSA that consists of three gas sensors for three toxic gases, it is possible to produce a large data set based on response curves, which subsequently can be evaluated and interpreted using statistical tools like cluster analysis and regression analysis. Cluster analysis namely hierarchical cluster analysis (HCA) and principal component analysis (PCA) gives very good classification among each gas. For data analysis, three variables namely sensor response, gas concentration and total response-recovery time have been measured from the response curve and used for data interpretation.
3.3.1 Cluster Analysis
This approach helps to identify the similarities present between the observations or variables and group them into clusters. For this, a measure of “closeness” or “similarity” is identified and corresponding similar items are linked together. Here, proximity is usually measured by the separation between observations in the p-dimensional space of the variables. In this study, Euclidean distances and Ward's linkage criterion are used to cluster the data. Ward's procedure unifies the clusters such that the size does not increase too much and the clusters are as homogeneous as possible [45]. The result is a dendrogram that indicates the similarities between the observations by comparing the distance between the branches of the binary tree i.e. the similarity is greater when the distance is smaller. This method is suitable for grouping the entities. The grouping continues until all the observations are converted into a single cluster. The following equation describes the distance between two observations, namely x and y:
Figure 14 shows the dendrogram as calculated using Ward’s method and Euclidian distance metric. The dendrogram could group the data into three sub groups. Each major subgroups are separated using three colour blocks. The dendrogram helps to identify the sequence of joints between clusters. Usually, lines are drawn connecting the clusters at each step with vertical axis representing the distance between the clusters. At the start, each of the gases forms a separate cluster. The entire procedure is continued till clubbing of the data into a single cluster is accomplished.
3.3.2 Principal component analysis (PCA)
PCA is generally used to make data, easy to explore and visualize. It helps to transforms a group of correlated variables into a group of uncorrelated variables [46, 47]. It often brings out the strong patterns in a data set, while decomposing the eigenvectors referred to as the principal components (PCs). Mostly, the original data set say matrix A (M x N), which is on the different range of scale is first standardized. The co-variance matrix finds the variance between the dimensions and then Eigen decomposition finds Eigenvalues and Eigenvectors. Herein, the Eigenvectors are nothing but the principal components. These form the new basis in the new coordinate system representing the data. The variance corresponding to the first principal component would be greater than that of the other principal components.
Figure 15a shows the scree plot, which is a demonstration of eigenvalues versus component number. The eigenvalues are ordered in a sequence from higher to the lowest value. Thus, the first component explains much of the variability, which decreases with the component number. The Eigenvalues corresponding to the first three principal components were 1.26, 0.99 and 0.73, respectively. Using the first three PCs the data can successfully be visualized with 100% of the variance as shown in Fig. 15b. There is an excellent clustering of three classes which implies that each observation from each class, fit into the feature space of corresponding classes. There is no need to add any other principal components so as to preserve any relevant information of the original data. It was visually examined that a very good separation between oxidizing gas, NO2 and reducing gases H2 and H2S exists. The separation boundaries between reducing gases namely H2 and H2S are very narrow and towards the same direction, which implies PCA hardly discriminated them. Contrarily, a very good discrimination between reducing and oxidizing gases is observed. Figure 16a shows the percent of variance and cumulative percentage of variance corresponding to each PCs. The first three components altogether can give 100 percentage of variance, which is shown as cumulative percent. The percentage of variance given by each PCs are shown in the figure, which can be determined by the ratio of eigenvalue that corresponds to the particular component to the total value of eigenvalue and multiplied by 100. The cumulative percentage is obtained by summing up the consecutive variances. Figure 16 (b) shows the correlation of component weights with respect to variables namely sensor response, total time and gas concentration. For gas concentration, the first three PCs show a positive correlation, whereas for total time the first PC, PC1 shows negative correlation, while other two shows a positive correlation. For sensor response, the first two PCs shows a positive correlation and PC3 shows a negative correlation. The corresponding numerical values observed are also listed in the figure. The visualization of the achieved distinct separation between H2S, H2 and NO2 is shown in Fig. 15b.
4 Conclusion
Chemiresistive E-nose comprising pristine, heterostructure ZnO NWs-PdO and ZnO NWs-Pt has been successfully developed and demonstrated for its efficacy towards discrimination of three gases namely H2, H2S and NO2. The incorporation of sensitizers into the ZnO permits an excellent sensitivity towards the test gases. XPS results revealed that Pd and Pt get incorporated as p-type PdO and metallic Pt, respectively. Consequently, the sensor surface is characterized by the presence of random nano p–n junctions and nano-Schottky junctions. The data repository was created utilizing gas concentration, sensor response and the total time taken towards different gas concentrations. Utilizing statistical implements namely PCA and HCA, the discrimination of H2, H2S and NO2 gases has been successfully accomplished. 3D PCA discriminates the three gases with a percentage of variance of 42.32, 33.26 and 24.20%, corresponding to the first three components namely PC1, PC2 and PC3, respectively. HCA dendrograms achieved using utilizing Ward’s method and Euclidian distance metric, groups the data into the three different clusters signifying a good discrimination of H2, H2S and NO2. The adsorption studies of target gases indicated that Pt- and Pd-incorporated ZnO NWs follow the Elovich model. This was further corroborated using the pseudo-second-order equations. The work function values for Pd-incorporated samples were found to decrease and increase by 450 and 40 meV, upon exposure to H2S and NO2 gases, respectively. And for Pt-incorporated samples, work function was found to decrease and increase by 160 and 50 meV, upon exposure to H2S and NO2 gases, respectively. Overall, our results indicated that the MSA based on ZnO NWs could be tuned to develop e-nose for simultaneous detection of H2, H2S and NO2 around and below their safety limit values. Hence, they seek practical utilization in E-noses to be employed in applications such as heavy water plants, oil and process industries and mines. Further, attempt would be to investigate the efficacy of the developed e-nose towards identification of gases in a mixture and is underway.
Data availability
Data will be made available on request.
References
N.S. Ramgir, N. Datta, M. Kaur, S. Kailasaganapathi, A.K. Debnath, D.K. Aswal, S.K. Gupta, Metal oxide nanowires for chemiresistive gas sensors: Issues, challenges and prospects. Coll. Surf. A 439, 101–116 (2013)
K.R. Sinju, N.S. Ramgir, A. Pathak, A.K. Debnath, K.P. Muthe, Multiple sensor array based on ZnO nanowires for electronic nose applications towards toxic gases. AIP Conf. Proc. 2265, 030282 (2020)
M.S. Haseman, H. Gao, K. Duddella, L.J. Brillson, Electrochemical Sensing of Uric Acid with Zinc Oxide Nanorods Decorated with Copper Oxide Nanoseeds. ACS Appl. Mater. Interfaces. 15(25), 30944–30955 (2023)
S. Masrat, V. Nagal, M. Khan, A. Ahmad, M.B. Alshammari, S. Alam, U.T. Nakate, B.I. Lee, P. Mishra, K.S. Bhat, R. Ahmad, Electric Field Manipulation of Defects and Schottky Barrier Control inside ZnO Nanowires. ACS Appl. Nano Mater. 6(18), 16615–16624 (2023)
N. S. Ramgir, S. J. Patil, ZnO Nanowires: An Excellent Nanomaterial for Nanotechnology, Encyclopedia of Semiconductor Nanotechnology, January 2017, ca. 4210 pages, ISBN: 1–58883–199-X.
F. Xu, W. Li, S. Sun, A. Zhong, X. Cheng, J. Shi, Z. Li, J. Li, W. Zhang, X. Wang, Y. Ma, A. Ho-Pui Ho, Expanding selectivity functionality of a ZnO nanotetrapod-based volatile organic compound sensor using au nanoparticle decoration. ACS Appl. Nano Mater. 6(10), 8335–8345 (2023)
K. Khamfoo, M. Punginsang, K. Inyawilert, A. Wisitsoraat, A. Tuantranont, C. Liewhiran, Effect of PdO-PdO2 core–shell nanocatalysts on hydrogen sensing performances of flame-made spinel Zn2SnO4 nanoparticles. Appl. Surf. Sci. 586, 152817 (2022)
P.K. Panigrahi, B. Chandu, Nagaprasad puvvada, recent advances in nanostructured materials for application as gas sensors. ACS Omega 9(3), 3092–3122 (2024)
J. Chao, H. Yu, K. Zhang, Y. Zhou, D. Meng, Y. Sun, Integration of ZnO and Au/ZnO nanostructures into gas sensor devices for sensitive ethanolamine detection. ACS Appl. Nano Mater. 6(7), 5994–6001 (2023)
Z. Wang, M.R. Bockstaller, K. Matyjaszewski, Synthesis and applications of ZnO/Polymer nanohybrids. ACS Mater. Lett. 3(5), 599–621 (2021)
V.S. Siril, K.N. Madhusoodanan, S.K. Rajan, G. Louis, Study on ammonia gas sensing property of hydrothermally synthesized pure ZnO nanorods. AIP Conf. Proc. 1849, 020033 (2017)
M.J. Ahemad, D.-S. Kim, T.D. Le, L.R. Nagappagari, G.-J. Oh, G.-S. Shin, U. Ahmad, S.A. Akbar, Y.T. Yu, Thermally stable AgPd@ZnO bimetallic alloy nanoparticles for ethanol sensors with long-term stability. ACS Appl. Nano Mater. 5(12), 18568–18580 (2022)
K.R. Sinju, B. Bhangare, A. Pathak, S.J. Patil, N.S. Ramgir, A.K. Debnath, D.K. Aswal, ZnO nanowires-based e-nose for the detection of H2S and NO2 toxic gases. Mater. Sci. Semicond. Proc. 137, 106235 (2022)
N.S. Ramgir, K.R. Sinju, B.B. Bhangare, A.K. Debnath, Electronic nose based on chemiresistive sensors for toxic gas detection. J. Mater. Nanosci. 9(2), 79–90 (2022)
N.S. Ramgir, C.P. Goyal, D. Goyal, S.J. Patil, H. Ikeda, S. Ponnusamy, K.P. Muthe, A.K. Debnath, NO2 sensor based on Al modified ZnO nanowires. Mater. Sci. Semicond. Process. 134, 106027 (2021)
N.S. Ramgir, R. Bhusari, N.S. Rawat, S.J. Patil, A.K. Debnath, S.C. Gadkari, K.P. Muthe, TiO2/ZnO heterostructure nanowire based NO2 sensor. Mater. Sci. Semicond. Process. 106, 104770 (2020)
N.S. Ramgir, P.K. Sharma, N. Datta, M. Kaur, A.K. Debnath, D.K. Aswal, S.K. Gupta, Room temperature H2S sensor based on Au modified ZnO nanowires. Sens. Actuators B 186, 718–726 (2013)
N. Datta, N.S. Ramgir, S. Kumar, P. Veerender, M. Kaur, S. Kailasaganapathi, A.K. Debnath, D.K. Aswal, S.K. Gupta, Role of various interfaces of CuO/ZnO random nanowires network in H2S sensing: an Impedance and Kelvin probe analysis. Sens. Actuators B 202, 1270–1280 (2014)
B. K. Bhangare, K. R. Sinju, S. J. Patil, Niranjan S. Ramgir, A. K. Debnath, Chapter-2, Introduction to nano E-nose, in Nanotechnology-based E-Noses: Fundamentals and Emerging Applications, Ed., T. A. Nguyen, M. Bilal, M. Ahmadi, R. Gupta, Elsevier, (2023), ISBN: 9780323911573. Copyright: © Woodhead Publishing, pg. 29–56.
N. S. Ramgir, K. R. Sinju, A. K. Debnath, S. J. Patil, Chapter 19 - Nanotechnology-based E-nose for smart manufacturing, in Nanosensors for Smart Manufacturing, Micro and Nano Technologies, 2021, pp. 417–444. ISBN: 9780128233580, Publisher: Elsevier.
S.K. Youn, N.S. Ramgir, C. Wang, K. Subannajui, V. Cimalla, M. Zacharias, Catalyst-free growth of ZnO nanowires based on topographical confinement and preferential chemisorption and their use for room temperature CO detection. J. Phys. Chem. C 114, 10092–10100 (2010)
N.S. Ramgir, Y. Yang, M. Zacharias, Nanowire-based sensors. Small 6, 1705–1722 (2010)
B. Bhangare, N.S. Ramgir, A. Pathak, K.R. Sinju, A.K. Debnath, S. Jagtap, N. Suzuki, K.P. Muthe, C. Terashima, D.K. Aswal, S.W. Gosavi, A. Fujishima, Role of sensitizers in imparting the selective response of SnO2/RGO based nanohybrids towards H2S, NO2 and H2. Mater. Sci. Semicond. Proc. 105, 104726 (2020)
B. Bhangare, K.R. Sinju, N.S. Ramgir, S. Gosavi, A.K. Debnath, Noble metal sensitized SnO2/RGO nanohybrids as chemiresistive E-nose for H2, H2S and NO2 detection. Mater. Sci. Semicond. Proc. 147, 106706 (2022)
S. Kailasa Ganapathi, M. Kaur, M. Shaheera, A. Pathak, S.C. Gadkari, A.K. Debnath, Highly sensitive NO2 sensor based on ZnO nanostructured thin film prepared by SILAR technique. Sens. Actuators B 335, 129678 (2021)
A. Ievtushenko, O. Khyzhun, I. Shtepliuk, V. Tkach, V. Lazorenko, G. Lashkarev, X-ray photo electron spectroscopy study of nitrogen and aluminum. Nitrogen-Doped ZnO Films, Acta Phys. Polonicaa 124, 5 (2013)
X. Chen, Y. Shen, P. Zhou, S. Zhao, X. Zhong, T. Li, C. Han, D. Wei, D. Meng, NO2 sensing properties of one-potsynthesized ZnO nanowires with Pd functionalization. Sens. Actuators B 280, 151–161 (2019)
H. Zhang, S. Yu, C. Chen, J. Zhang, J. Liu, P. Li, Effects on the structure, surface oxygen defects and humidity performance of Au modified ZnO via hydrothermal method. Appl. Surf. Sci. 486, 482–489 (2019)
M. Kashif, M.E. Ali, S.M. Usman, U.H. Ali, Sol–gel synthesis of Pd doped ZnO nanorods for room temperature hydrogen sensing applications. Cer. Int. 39, 6461–6466 (2013)
J. Huang, J. Zhou, Z. Liu, X. Li, Y. Geng, T. Xiaoquig, Y. Du, Z. Qian, Enhanced acetone-sensing properties to ppb detection level using Au/Pd-doped ZnO nanorod. Sens. Actuators B 310, 127129 (2020)
S.M. Majhi, H.-J. Lee, H.-N. Choi, H.-Y. Cho, J.-S. Kim, C.-R. Lee, Y.-T. Yu, Construction of novel hybrid PdO–ZnO p–n heterojunction nanostructures as a high-response sensor for acetaldehyde gas. Cryst. Eng. Comm. 21, 5084–5094 (2019)
C. Dablemont, P. Lang, C. Mangeney, J.Y. Piquemal, V. Petkov, F. Herbst, G. Viau, FTIR and XPS study of Pt nanoparticle functionalization and interaction with alumina. Langmuir 24, 5832–5841 (2008)
K.N. Han, C.A. Li, M.P.N. Bui, X.H. Pham, B.S. Kim, Y.H. Choa, G.H. Seong, The development of Pt/TiO2 nanohybrids-modified SWCNT electrode for sensitive hydrogen peroxide detection. Sens. Actuators B 174, 406 (2012)
T.N.T. Oliveira, C.A. Zito, T.M. Perfecto, G.M. Azevedo, D.P. Volanti, ZnO twin-rods decorated with Pt nanoparticles for butanone detection. New J. Chem. 44, 15574–15583 (2020)
X.-Y. Wu, L.-Y. Zhu, J. Sun, K.-Y. Zhu, X.-Y. Miao, M.-Y. Liu, X.-F. Zhao, H.-L. Lu, Pt nanoparticle-modified SnO2–ZnO core-shell nanosheets on microelectromechanical systems for enhanced H2S detection. ACS Appl. Nano Mater. 5(5), 6627–6636 (2022)
Z. Shao, L. Wen, D. Wu, X. Zhang, S. Chang, S. Qin, Pt/ZnO Schottky nano-contact for piezoelectric nanogenerator. Phys. E 43, 173–175 (2010)
Y. Xu, C. Cheng, S. Du, J. Yang, B. Yu, J. Luo, W. Yin, E. Li, S. Dong, P. Ye, X. Duan, Contacts between two- and three-dimensional materials: ohmic, schottky, and p–n heterojunctions. ACS Nano 10(5), 4895–4919 (2016)
S. Ranwa, M. Kumar, P.K. Kulriya, M. Fanetti, M. Valant, M. Kumar, Improvement in the sensing response of nanocrystalline ZnO-based hydrogen sensor: effect of swift heavy ion irradiation. IEEE Sens. J. 16, 7586–7592 (2016)
R. Bhusari, J.-S. Thomann, J. Guillot, R. Leturcq, Oxygen adsorption and desorption kinetics in CuO Nanowire bundle networks: implications for MOx-based gas sensors. ACS Appl. Nano Mater. 5(8), 10248–10257 (2022)
Z. Wang, T. Zhang, C. Zhao, T. Han, G. Lu, Anchoring ultrafine Pd nanoparticles and SnO2 nanoparticles on reduced graphene oxide for high-performance room temperature NO2 sensing. Coll. Interface Sci. 514, 599–608 (2018)
M. Yadav, M. Kumar, S. Chaudhary, K. Yadav, A. Sharma, A review on chemiresistive hybrid zinc oxide and nanocomposites for gas sensing. Ind. Eng. Chem. Res. 62(29), 11259–11278 (2023)
S. Altun, E.B. Orman, Z. Odabas, A. Altindal, A.R. Ozkaya, Gas sensing and electro chemical properties of tetra and octa 2H-chromen-2-one substituted iron(II) phthalocyanines. Dalton Trans. 44, 4341–4354 (2015)
A.K. Debnath, S. Samanta, A. Singh, D.K. Aswal, S.K. Gupta, J.V. Yakhmi, Parts per-billion level chlorine sensors with fast kinetics using ultra-thin cobalt phthalocyanine films. Chem. Phys. Lett. 480, 185–188 (2009)
D.V. Ponnuvelu, J. Dhakshinamoorthy, A.K. Prasad, S. Dhara, M. Kamruddin, B. Pullithadathil, Geometrically controlled Au-decorated ZnO heterojunction nanostructures for NO2 detection. ACS Appl. Nano Mater. 3(6), 5898–5909 (2020)
D. Granato, J.S. Santos, G.B. Escher, B.L. Ferreira, R.M. Maggio, Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: a critical perspective. Trends Food Sci. Tech. 72, 83–90 (2018)
M. Hubert, S. Engelen, Fast cross-validation of high-breakdown resampling methods for PCA. Comp. Stat. Data Anal. 51, 5013–5024 (2007)
T. Jolliffe, J. Cadima, Principal component analysis: a review and recent developments. Trans. R. Soc. A 374, 20150202 (2016)
Acknowledgements
Author K. R. Sinju thanks Council of Scientific and Industrial Research – University Grants Commission (CSIR-UGC) for the senior research fellowship (SRF). Authors also thank Dr. P. K. Patro for his help with SEM/EDS measurements.
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Contributions
K. R. Sinju: Conceptualization, Data curation, Formal analysis, Original draft, Methodology and Software; Nida Khan: Data curation and Characterization; A. K. Debnath: Supervision and Resources; and N. S. Ramgir: Conceptualization, Methodology, Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sinju, K.R., Khan, N., Debnath, A.K. et al. Electronic nose based on Pd- and Pt-incorporated ZnO nanowires: a case study. J Mater Sci: Mater Electron 35, 1019 (2024). https://doi.org/10.1007/s10854-024-12784-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12784-1