Abstract
n-TPA-IFA organic material was synthesized and deposited on p-Si by spin coating method to produce n-TPA-IFA/p-Si heterojunction diode. We determined that the dielectric constant and energy band gap of TPA-IFA organic material were 3.91 and 3.37 eV by DFT/B3LYP/6-311G(d,p) method using on Gaussian 09 W, respectively, and the carrier type of TPA-IFA organic semiconductor material was also n-type at room temperature from temperature-dependent hall effect measurements. Using forward and reverse bias I–V measurements in the dark and under various light intensities, we examined the key electrical characteristics of the n-TPA-IFA/p-Si heterojunction diode, including \({\Phi }_{b}\) and \(n\). It was determined that the rectification ratio (RR) was approximately 104. The reverse current's observed increasing behavior with increasing light indicates that the produced heterojunction diode can be utilized as a photodiode, detector, or sensor. The values of n, \({\Phi }_{b}\), and Io were determined using I–V data in dark as 1.34, 0.91 eV, and 7.25 9 10–12 A, respectively. On the other hand, when the n-TPA-IFA/p-Si heterojunction diode is exposed to 100 mW/cm2 illumination, these parameters were obtained as 1.85, 0.80 eV, and 5.11 9 10–10 A, respectively. Photovoltaic parameters such as short circuit current (Isc) and open circuit voltage (Voc) were determined as 0.018 mV, 0.08 A and 0.050 mV, 0.29 A under 20 mW/cm2 light and 100 mW/cm2 light intensity, respectively. The photodetector properties of n-TPA-IFA/p-Si heterostructure were explored at different light intensities, and the photoresponsivity (R), photosensitivity (PS), specific detectivity (\({D}^{*}\)), and linear dynamic range (LDR) of the heterojunction changed with reverse voltage and light intensity. It was found that as light intensity increased, the linear dynamic range (LDR), a crucial characteristic for image sensors, increased as well (10.15 dB and 18.84 dB for 20 and 100 mW/cm2).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The potential primary advantages of organic material-based electronic and photoelectrical devices, such as their low cost and easy preparation processes, versatility, and ability to cover large areas, have led to a significant increase in research interest in recent decades. Due to their superior photoconductive capabilities and exceptional thermal and chemical stability, organic semiconducting compounds have been used extensively in photoelectric and electronic devices which are used in optoelectronic devices. Organic D-π-A conjugated systems, composed of donor (D) and acceptor (A) groups linked by π-bridges, have received significant attention in various scientific fields due to their unique photophysical properties. Because of their remarkable optical properties, these hybrid D-π-A skeletons have been widely used in materials chemistry for applications such as organic light-emitting diodes (OLEDs), dye-sensitized solar cells (DSSCs), nonlinear optical materials, fluorescence imaging, and memory [1,2,3].
Thus far, the literature has documented the utilization of organic dyes, specifically D-(π-A), D-π-A, D-D-π-A, D-A-π-A, and (D-π-A). One of the dyes that stands out is the D-A-π-A conjugate system, which offers several significant benefits [4,5,6]. These include broad absorption and emission bands in the UV–Visible region, effective intramolecular charge transfer, easily adjustable molecular orbital energy levels, optoelectronic properties, and narrow band gap energy. Therefore, utilizing “D-A-π-A” organic sensitizers has been considered a potential strategy for enhancing cell efficiency and optimizing photovoltaic performance in optoelectronic devices, as opposed to conventional “D-π-A” sensitizers.
Organic–inorganic (OI) heterojunctions are one of the electronic and photoelectrical device classes. They are widely studied to use the advantages of both organic and inorganic materials in a single structure [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. A metal/organic material/semiconductor (MOmS) heterojunction is created by adding a thin organic layer in between the metal/Si contacts [7,8,9]. A thin organic substance between the metal and semiconductor can form a dipole layer, allowing the Schottky barrier height of the junctions to be controlled [10,11,12]. It has been reported in many articles in the literature that organic semiconductor films on Si semiconductor substrate improve the electronic and optoelectronic properties of metal–semiconductor contacts [13,14,15,16,17].
Triphenylamine (TPA) derivatives, which serve as hole transfer materials, have gained popularity in fabricating electroluminescent devices across multiple industries [18,19,20,21]. TPA compounds offer numerous benefits, including their photoconductive and light-emitting characteristics, adjustable electronics, and straightforward processing. Due to their exceptional thermal and optical stability, they are well-suited for altering donor-π-acceptor systems [22,23,24,25]. Moreover, TPA derivatives' impressive hole drift mobility enhances their potential for optoelectronic devices [26,27,28,29,30]. Triphenylamine-derived compounds have also been synthesized for several optoelectronic applications. Few of these, however, have had their structural and optical characteristics examined about applying TPA compound as an interfacial layer on metal/p-Si or metal/n-Si thin films. Schiff base compounds have become a topic of interest as organic semiconductors due to their unique electrical and optical properties. Schiff base molecules are often used to create Schottky barrier diodes, but their use is limited due to hydrolysis during device fabrication [31, 32]. The use of Schiff base compounds in creating Schottky barrier diodes is greatly appreciated due to their straightforward synthesis, low cost, and minimal time requirement. However, while triphenylamine and its derivatives have been used as linkers to create hybrid materials, limited research has been conducted on the impact of TPA-based Schiff base compounds as organic interfacial layers on the electronic parameters of Schottky devices. Therefore, more research on their optical and electrical properties is required to explore the potential applications of TPA-based Schiff base compounds in optoelectronics.
In this work, we report for the first time n-type TPA-IFA (“D-A-π-A”) compound, in which triphenylamine is used as a donor, the C = N imine part is an acceptor, benzene is a π-spacer, and ester is an acceptor/anchor. Then, n-TPA-IFA was used as a new synthesized organic interfacial layer to modify or improve the photodiode parameters and device performance by generating dipoles at the interface. To the best of the author's knowledge, TPA-IFA thin film has not yet been employed as an interfacial layer in heterojunction and optoelectronic applications in the literature. For this reason, the TPA-IFA organic molecule interface heterojunction devices, n-TPA-IFA/p-Si heterojunction diode, were created, and the optoelectronic and electronic characteristics of the fabricated devices were investigated by using current–voltage (I–V) measurements under both different light intensities and dark conditions.
2 Experimental
2.1 Synthesis and characterization of the TPA-IFA
The synthesis pathway for TPA-IFA is shown in Scheme 1. Compound 2 was synthesized from triphenylamine (1) by using Vilsmeier–Haack formylation reaction [33]. To a solution of compound 2 (1.0 mmol) in THF (10 mL), dimethyl 5-aminoisophthalate (3) (2.1 mmol) was added. Then, the mixture was refluxed at 80 °C for 12 h, then the THF was removed via a rotary evaporator. The obtained solid was subjected to filtration, drying, and subsequent recrystallization using ethanol, yielding a pure compound. 1H NMR (400 MHz, CDCl3) δ: 8.54 (s, 2H), 8.48 (s, 1H), 8.08–8.06 (m, 4H), 7.64 (d, J = 8.5 Hz, 4H), 7.40–7.38 (m, 2H), 7.28–7.21 (m, 5H), 3.99 (s, 12H). 13C NMR (100 MHz, CDCl3) δ: 166.5, 161.6, 152.0, 149.9, 146.7, 131.4, 130.4, 130.1, 126.7, 126.2, 125.8, 124.2, 52.3.
2.2 The device fabrication
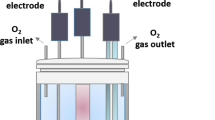
Silicon wafer with a 2-inch diameter p-type, orientation (100), 400 μm thickness, 1–10 Ω.cm was cut to suitable sizes with a diamond-tipped pen. First, the p-Si wafer was cleaned with acetone and methanol for 10 and 5 min, respectively, an ultrasonic bath at 50 °C. RCA1 and RCA2 procedures were used for p-Si wafer cleaning. H2O2: H2O solutions were prepared with a mixing ratio of 10:6. The RCA1 cleaning process was used to remove an organic layer; in this process, 5 ml of NH3 (%35) and 25 ml of H20 were mixed and heated to 70 °C. Then, 5 ml of H2O2:H2O solution was added to this solution. The Si substrate was put in the solution and kept for 15 min. RCA2 process was used to remove an inorganic layer; for this cleaning process, 5 ml of HCl (%27) and 25 ml H2O were mixed and heated to 70 °C. Then 5 ml of H2O2:H2O solution was added to this solution. The Si substrate was put in the solution and kept for 15 min. To remove the oxide layer, the Si wafer was placed in a 2% HF solution for 3 min. Then it was rinsed with H2O. The Al ohmic contact was deposited with the help of a tungsten crucible under 10–6 bar pressure with a thermal evaporation system for the p-Si wafer. Then, the Si wafer was annealed for five minutes at 570 °C in a pure argon atmosphere. Also, a soda-lime glass substrate was cleaned with chloroform, deionized water, methyl alcohol, acetone, and propanol for 10 min in an ultrasonic bath for the Hall measurement. 40 mg TPA-IFA was dissolved in 10 ml N, N-Dimethylformamide (DMF), and the solution was mixed for 10 min on a magnetic stirrer. A yellowish-clear solution was obtained. TPA-IFA solution was grown on the p-Si wafer and glass substrate by using the spin-coating method at 1000 rpm for 30 s. 1.15-mm-diameter Al dots contacts were deposited with the same conditions as ohmic contact with a thermal evaporation system for p-Si wafer for electrical I–V measurements (Fig. 1). The samples grown on a glass substrate for Hall measurement were cut into a square of 7 mm by 7 mm, and their contacts were done from each corner with the help of silver paste.
Current–voltage measurements were taken between − 3 V and + 3 V step by 0.01 V at dark and under different light illumination conditions such as 20 mW.cm−2, 40 mW.cm−2, 60 mW.cm−2, 80 mW.cm−2, and 100 mW.cm−2. Hall measurements were taken by van der Pauw technique at 0.5 T magnetic field between 100 and 400 K step by 50 K (Fig. 2).
3 Results and discussion
3.1 Theoretical studies
To obtain comprehensive knowledge about the compound's electronic structure, conductivity, and optimal molecular geometry in its ground state, the synthesized TPA-IFA was first computationally evaluated using DFT. The Gaussian 09 program suite software was used to obtain various properties of our compound, including the optimal structural geometry, analysis of the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO), MEP surface analysis, molecular static isotropic polarizability volume, and molar volume [34]. Figure 3 shows the simulated HOMO and LUMO drawings of the molecule at the gas phase using the DFT-B3LYP/6-311G (d,p) computational level. As seen, the HOMO of the Terp-Fc in Fig. 3 is mainly delocalized on three substituted phenyls connected to nitrogen, while the LUMO is delocalized on two phenyls, C = N imine, and isophthalate (Ph(CO2Et)) moieties. These results suggest that the main interaction between the charged surface and the Terp-Fc molecule occurs in this specific region. The LUMO and HOMO states of a molecule are responsible for its electron-capturing and electron-donating features, respectively. The HOMO–LUMO gap expresses the charge transfer interaction within a molecule in a given compound by representing the difference between the HOMO and LUMO energy levels. The HOMO energy correlates with the ionization potential while the LUMO energy correlates with the electron affinity. The computed results of the aforementioned molecular descriptors are given in Table 1 based on these values. As seen, the energy band gap in our molecule was calculated theoretically to be 3.37 eV. As is well known from the literature, \(\Delta\) E offers details about the compound's conductivity. Since semiconductors' \(\Delta\) E range is between 0.5 and 3.5 eV, TPA-IFA compound may exhibit semiconducting properties. Moreover, the Clausius–Mossotti equation, which provides a connection between a material's molar volume and polarizability and its dielectric constant, is known to be stated as follows [35]:
where \({\varepsilon }_{r}\) is the dielectric constant, VM is the molar volume of material, and NA is Avogadro’s constant. Also, the \({\alpha }{\prime}\) is the polarizability volume in terms of α/4π \({\varepsilon }_{0}\). For TPA-IFA at the isolated gas phase state, the compound's dielectric constant was found as 3.91. The chemical hardness is a highly essential reaction property to the subject as the measure of the resistance of chemical species to electron cloud polarization or deformation in the electric field. The chemical hardness is also the measure of the stability and reactivity of the molecules. Typically, the molecule is soft when the energy gap is the lowest, has high chemical reactivity, low kinetic capacity, and the least high resistance to charge transfers and electron restraint and distribution. Since the Terp-Fc molecule has a relatively low energy band gap, it is considered theoretically a soft substance because it has a value close to organic semiconductors.
3.2 Synthesis of TPA-IFA
Scheme 1 displays the chemical routes used to synthesize the TPA-IFA compound. Firstly, 4,4'-(phenylazanediyl)dibenzaldehyde (2) was obtained through Vilsmeier formylation of the triphenylamine [33]. For this, triphenylamine (1), which was commercially available, was treated with a POCl3 in DMF for 12 h to give compound 2 in good yield (98%). In a further reaction step, compound 2, treated with the two equimolar amounts of dimethyl 5-amino isophthalate, formed TPA-IFA as a major product with a yield of 55%.
The 1H NMR spectrum of compound TPA-IFA in CDCl3 is presented in Fig. 4. The structural assignment of TPA-IFA was based on the appearance and disappearance of the 1H NMR signals at δ = 8.54 for the CH = N protons peaks of the imine groups and at δ = 9.88 for the aldehyde protons of compound 2, respectively. As expected, the characteristic CH3 protons (12H) peaks were observed at 3.99 ppm. In addition, other aromatic ring protons (12H) resonate as singlets at 8.48 (2H) and 8.08 (4H), doublet at 7.64 (d, 4H), and multiplets at 7.38–7.40 (m, 2H), and 7.21–7.28 (m, 5H). In the 13C NMR spectra of compound TPA-IFA, the signals at 166.5 ppm were assigned to carbons of C = O groups, whereas the imine carbons (C = N–) were observed at 161.6 ppm. The NMR data were in very good agreement with the structure of the TPA-IFA molecule.
3.3 The hall measurement
Figure 5 shows a color mapping plot of mobility for TPA-IFA. Hall measurements were taken at 0.5 T magnetic field between 100 and 400 K step by 50 K. The sample showed p-type characterization at 100 K and 150 K, while it showed n-type characterization between 200 and 400 K. The carrier charge density type of the sample changed between 150 and 200 K. The mobility of TPA-IFA varied depending on temperature. The mobility increased with increasing temperature between 100 and 250 K. Then it is decreased for 300 K and 350 K. Lastly, it increased to 400 K. The temperature with the highest mobility value is 250 K. According to these results, the TPA-IFA sample shows n-type behavior at room temperature.
3.4 Structural analysis
A surface morphological analysis of the prepared thin film was done using a scanning electrode microscope (SEM) operated at an accelerating voltage of 1 kV to analyze the thin film's microstructure. Figures 6 a, b, and c show SEM images of the TFA-IFA film on Si crystal, its corresponding energy-dispersive x-ray spectroscopy (EDS) spectrum, and elemental mapping of TFA-IFA/n-Si structure, respectively. The mapping on the thin film shows a homogeneous distribution of Si, O, and C. SEM images of the surface of the diode in Fig. 6b were used to provide EDS analysis. According to the EDS analysis, silicon from the Si wafer, oxygen, and carbon elements from the organic structure are distributed quite homogeneously on the surface. When the mass ratio of silicon from the substrate is examined, it can be said that it is dominant compared to other molecules.
3.5 Current–voltage characterization of n-TPA-IFA/p-Si heterojunction
Figure 8 illustrates the current–voltage (I–V) characteristics of the n-TPA-IFA/p-Si heterostructure under dark and different light intensities. The plots clearly demonstrate that all measurement curves exhibit behavior similar to that of a diode. Rectification behavior can be seen from all curves in Fig. 8, and the rectification rate in the dark is very high at \(\mp\) 3 V, and its value is 1.095 × 104. The rectification rates for dark and different light intensities at \(\mp\) 3 V are given in Table 2. The same graphs additionally demonstrate strong linear behavior at intermediate bias voltages that have distinct slopes, but at sufficiently large forward biases, they deviate from linearity, primarily due to the interface layer. Thermionic emission theory can analyze the I–V characteristics of this type of rectifying contact, and the parameters of the heterojunction can be calculated with the help of the following equation (to understand the formation of the n-TPA-IFA/p-Si heterojunction, see Fig. 7): [36,37,38]:
where the reverse saturation current (\({I}_{0}\)) is also given by the following equation [37]:
where \({A}^{*}\) is the effective Richardson constant, \(A\) is the active device area, \(n\) is the ideality factor, \({\Phi }_{b}\) is the barrier height, \(T\) is the temperature, \(q\) is the electronic charge, \(V\) is the applied voltage, \(k\) is the Boltzmann constant, and \({R}_{sh}\) is the saturation current.
The ideality factor (\(n\)) and barrier height (\({\Phi }_{b}\)) values of the fabricated heterojunction diode can be calculated by the following equations [39]:
From thermionic emission (for V ≥ 3kT⁄q) theory, the intercept and slope of the I–V plots of Fig. 8 are used to determine the \({I}_{0},{\Phi }_{b}\), and \(n\), respectively. Table 2 lists the computed values of \({I}_{0},{\Phi }_{b},\) and \(n\) values for the n-TPA-IFA/p-Si heterojunction diode in response to varying light intensities and dark. Table 2 illustrates that the barrier height rises with light intensity, and the ideality factor falls comparatively. As is known, the asymmetric and nonlinear behavior of the I–V curves are shown by the values of the rectification ratio (RR). As seen in Table 2, the decrease in RR as light intensity increases indicates a decrease in charge transport efficiency through the molecular junction. This could be due to increased scattering or interference effects as more molecules are packed closely together, reducing the ability to rectify current flow in one direction over the other. The performance of molecular diodes is still contact-limited, nevertheless, which poses a significant obstacle to the advancement of conductance and rectification ratio. Numerous rectification mechanisms in molecular diodes have been extensively explained, including molecular dipole [40], molecule conformation [41], and asymmetry in the molecule–electrode contact [42, 43]. But up until now, the majority of molecular diodes have only shown typical RRs between 102 and 105. Furthermore, high rectification factor and low ideality factor have been reported in monolayer molecular diodes with a thickness of approximately 3 nm [44]. For the fabricated n-TPA-IFA/p-Si heterojunction diode, the calculated experimental values of \({I}_{0},{\Phi }_{b}\), and \(n\) were 121 nA, 0.716 eV, and 3.01 in the dark and 26.1 nA, 0.755 eV, and 1.63 under 100 mW/cm2. The ideality factor value from Table 2 is greater than the ideal value of 1, indicating that the diode is not behaving ideally. The greater ideality factor value indicates the existence of interface states, an organic TPA-IFA film on the silicon crystal, and series resistance in addition to the inhomogeneities of the Schottky barrier height [45, 46]. As seen in Fig. 8, the photocurrent rises as illumination intensity rises in the reverse bias voltages, confirming that the fabricated heterojunction diode exhibits both photovoltaic and photoconductive activity [47,48,49]. Also, the circuit diagram of the photodiode is given in Fig. 8 inset. The circuit consists of a parallel shunt resistance (Rsh)–diode connected in series resistance (Rs). Leakage current appeared under light illumination and is shown in the circuit diagram. Using a spin coating technique, Karataş and Berk created an Al/GO:PTCDA/p-Si diode by depositing graphene oxide (GO) and perylene tetracarboxylic dianhydride (PTCDA) interfacial layers on p-type Si [50]. They investigated the photodiode characteristics of the generated diode in various lighting conditions and complete darkness. In the dark and at 100 mW/cm2, they determined the values of the n and \({\Phi }_{b}\) parameters to be 6.14–0.618 eV and 5.82–0.624 eV, respectively. The electrical characteristics of the Al/P3HT/p-Si Schottky diode were investigated by Ocaya et al. under various lighting conditions [51]. They demonstrated that when illumination intensity increased, the ideality factor decreased for dark and 100 mW/cm2 from 13.58 to 13.26 and the barrier height increased from 0.649 to 0.658 eV, respectively.
The resistance found in the metal-interfacial interface is called the series resistance in the heterojunction diode. The heterojunction diode's functionality and its capacity to regulate the flow of electrical current may be impacted by this resistance [29]. Numerous elements, including imperfections in the interfacial layer, metal or semiconductor layer contaminants, and variations in the interfacial layer's thickness and quality, can contribute to series resistance. Multiple ways are available for calculating series resistance in a heterojunction diode [45, 52]. The resistance (\({R}_{i}\)) values of the heterojunction diode can be determined from Ohm’s Law (\({R}_{i}=d{V}_{i}/d{I}_{i}\)) both in dark and under different illumination intensities. \({R}_{i}\) versus voltage graphs of the n-TPA-IFA/p-Si heterostructure diode for varying the measurement light intensity are shown in Fig. 9. For sufficient lower and higher bias voltages, the values of \({R}_{i}\) approach nearly to a constant value; hence, the values of \({R}_{i}\) refer to the correct values of the shunt resistance (\({R}_{sh}\)) and series resistance (\({R}_{s}\)), respectively, both in the dark and under various levels of light. These values were calculated for the fabricated heterojunction diode and are given in Table 2. Because of the increasing current, particularly at reverse biases, the \({R}_{i}\) values declined almost regularly as the light intensity increased (Fig. 9, Table 2). For a certain type of light sensor application, this outcome is significant. These values are suitable for a heterojunction diode: 58–753 kΩ and 49–52 Ω are the levels of \({R}_{sh}\) and \({R}_{s}\) values.
Photocurrent (\({I}_{ph}\)), a reverse current, rises with increasing illumination intensities, as shown in Fig. 10. Photodiodes are devices that convert optical signals to electrical current. They have been the subject of extensive research in recent years. Based on light intensity, current measurements verify that the electricity produced photodiode demonstrates photovoltaic activity [53,54,55]. Through the process of the photovoltaic effect, light energy is transformed into electrical energy by photovoltaic materials. In this context, organic materials such as TPA-IFA thin-film heterojunction can provide several benefits. Photons that penetrate the n-TPA-IFA/p-Si heterojunction diode when it is exposed to light intensity cause electron–hole pairs to form in the semiconductor structures. As a result, a reverse bias voltage is applied to separate the produced electron–hole pairs, and the interface layer's injected holes provide a large photocurrent [56,57,58]. The following formula yields the photocurrent (\({I}_{ph}\)):
where \({I}_{dark}\) and \({I}_{light}\) represent the reverse bias current values at dark and under different light intensities, respectively. This indicates that because higher energy photons are more likely to overcome the energy barrier, at greater light intensities, the photocurrent likewise increases. The drift velocity of photogenerated electrons and holes at the TPA-IFA/p-Si film interface may cause a rise in photocurrent [59, 60].
Additionally, the interaction between the light intensity (\(P\)) and the photodiode's photocurrent (\({I}_{ph}\)) is shown as follows [59]:
where \(m\) is an exponent determining the photoconduction process and A is the proportionality constant. The n-TPA-IFA/p-Si heterojunction photodiode's log(\({I}_{ph}\))–log(\(P\)) plot is shown in Fig. 10, and the magnitude \(m\) was calculated from its slope, which was 0.621 at – 3.0 V. The photoconductive transient mechanism of the n-TPA-IFA/p-Si heterostructure was verified by the linearity seen in Fig. 10, making it a promising option for photodetection applications [61]. The value of the \(m\) can be used to identify the nature of the photoconducting mechanism. If this value falls between 0.5 and 1, it indicates that localized trap levels are continuously distributed [62, 63].
For an organic-based photodiode device to be utilized in optoelectronic applications, it is essential to ensure the stability of its photoresponse. The aim was accomplished via time-dependent transient photocurrent measurements of the fabricated diode that were conducted employing various illuminating levels, and the results are displayed in Fig. 11. Also, transient photocurrent measurements of the diode were carried out for additional photoresponse analysis. The transient current measurements provide insight into the current conduction mechanism and aid in the determination of several photodetector properties, including photosensitivity, responsivity, and detectivity, for variations in light intensity [57, 64]. As seen in Fig. 11, when the diode is illuminated, electrons contribute to the current, and the quantity of photogenerated charge carriers increases with illumination. Both the diode's current and the quantity of free electrons decrease when the light is turned off. As a result, the trap centers found in the TPA-IFA organic material determine the photoconducting characteristics of the diode. Moreover, the Ion/Ioff ratio of the Al/TPA-IFA/p-Si/Al diode is approximately 35.1, indicating a photoresponse feature. The results similarly allow us to infer that the photodiode demonstrates stable and reproducible photo-switching from high to low conductivity states.
To investigate the stability and reusability of the n-TPA-IFA/p-Si heterojunction structure, current–voltage measurements were also taken at different times such as 5, 10, 15, and 20 days. Figure 6 depicts the \(lnI-V\) curves of the n-TPA-IFA/p-Si heterojunction structure measured after 5, 10, 15, and 20 days. According to thermionic emission theory, barrier height and ideality factor values were calculated using the intercept and slope points of the curves in Fig. 12. These barrier height and ideality factor values were obtained as 0.859 eV-1.682 for 5 days, 0.855 eV-1.738 for 10 days, 0.862 eV-1.727 for 15 days, and 0.867 eV-1.727 for 20 days. When we look at both the current–voltage curves and the calculated diode parameters, we can say that the TFA-IFA/p-Si heterojunction structure is stable and can be reused. Furthermore, measurements of photocurrent were made across a broad 600-s time range. At 100 mW/cm2 of light, as shown in Fig. 12, the photocurrent value increased gradually to about 2.14 × 10–4 A and remained constant for 600 s. At different light intensities, a similar tendency is observed.
The semi-logarithmic I–V graphs for the n-TPA-IFA/p-Si heterojunction structure are displayed in Fig. 13 in the temperature range of 300–400 K with 25 K increments. It is evident from Fig. 13 that I–V measurements stabilize with rising temperatures. The barrier height and ideality factor values of the n-TPA-IFA/p-Si heterojunction structure were obtained as 0.743 eV-2.47 for 300 K, 0.708 eV-2.58 for 325 K, 0.682 eV-2.54 for 350 K, 0.684 eV-2.59 for 375 K, and 0.676 eV-2.60 for 400 K. When we look at both the current–voltage curves and the calculated diode parameters, we can say that the TFA-IFA/p-Si heterojunction structure is stable.
Through the utilization of semiconductors, photovoltaic devices can transform solar energy into electrical energy. Because of the photoelectric effect, these semiconductor structures can take in photons from the sun and then emit electrons as a result of the process. When photons have energies higher than or equal to the energy gap (hv ≥ Eg), they can cause additional electrons in the valence band (Ev) and trap levels to move to the conduction band (Ec) or trap levels, thus absorbing the photons. This process is known as photocurrent (\({I}_{ph}\)). Depending on the intensity of the light, this electron mobility results in the formation of the open circuit voltage (\({V}_{oc}\)) and short circuit current (\({I}_{sc}\)) at I = 0 A and V = 0 V, respectively. The characteristics of current density (\(J\)) against voltage of the n-TPA-IFA/p-Si heterojunction diode are shown in Fig. 14 under different light intensities (from 20 to 100 mW/cm2). The inset of Fig. 14 shows the characteristics of short circuit current density (\({J}_{sc}\)) and open circuit voltage (\({V}_{oc}\)) against the light intensity of the n-TPA-IFA/p-Si heterojunction diode. Also, the calculated values of short circuit current density (\({J}_{sc}\)) and open circuit voltage (\({V}_{oc}\)) with light intensity are illustrated in Table 3. It presents a notable rise in open circuit voltage (\({V}_{oc}\)) and short circuit current (\({J}_{sc}\)) values with increasing light intensity, as seen in Fig. 14. This is because a heterojunction diode will absorb more light and produce more photon carriers when the light intensity is increasing. Because the n-TPA-IFA/p-Si structure has photovoltaic characteristics, it can, therefore, be employed as a photodiode. Consequently, the research findings demonstrated that when light intensity increased, the photovoltaic characteristics increased. Similar to this, İmer and Ocak studied an p-Si/Sunset yellow/Al inorganic–organic heterojunction diode and found that, when exposed to 100 mW/cm2 of light, the diode functioned like a photodiode, with a maximum \({V}_{oc}\) of 0.375 V and an \({I}_{sc}\) of 22.5 \(\mu\) A [65]. Furthermore, based on Coumarin 30 organic material, Tombak et al. reported the photocurrent–voltage characteristic of an p-Si/Al diode; under 100 mW/cm2 illumination, it was stated to exhibit photovoltaic properties with a maximum \({V}_{oc}\) of 0.337 V and an \({I}_{sc}\) of 59 \(\mu\) A [66].
The following equations for photoresponsivity (R), photosensitivity (PS), specific detectivity (\({D}^{*}\)), and linear dynamic range (LDR) were used to determine the detector characteristics of the n-TPA-IFA/p-Si heterostructure diode [67, 68].
Here, \(A\) represents the detector's active area, and \(P\) represents the incident light density. One important metric that shows how a photodiode reacts to incoming light is photoconductive photoresponsivity (\(R\)) [69]. Its definition is as follows: it is the ratio of photocurrent generated to the optical power of incoming light. The variation of \(R\) for the photodiode with an applied reverse bias voltage at light intensities ranging from 20 to 100 mW.cm−2 is depicted in Fig. 12. The change of \(R\) depending on the light power applied at − 3 V is given in the inset of Fig. 15. The various values that were found range from 11.20 to 20.59 mA/W, which is advantageous for high-speed diode operation [70, 71]. \(R\) values depending on light intensity are also given in Table 3.
The ratio of the photocurrent to the dark one for the heterojunction diode is known as photosensitivity (\(PS(\%)\)). Measurements of photosensitivity characteristics as a function of light intensities are also made to determine whether the device is suitable for use with photodiodes. This crucial variable can also be employed to maintain the level of light-to-current conversion. Figures 16 display the reverse bias voltage profile of the photosensitivity (\(PS(\%)\)) of the n-TPA-IFA/p-Si heterostructure. The change of \(PS\left(\%\right)\) depending on the light power (\(P\)) applied at -3 V is given in the inset of Fig. 16. From the inset of Fig. 16, the values of \(PS(\%)\) were found to range from 321.76 to 875.44 for 20 and 100 mW/cm2, respectively. \(PS(\%)\) values depending on light intensity are also given in Table 3.
The primary parameter for a photodetector is its specific detectivity (D*), which is the minimum light intensity that a diode can detect. In other words, \({D}^{*}\) describes a photodetector's capacity to detect low-light signals. This parameter is based on the produced photodiode's noise and responsivity. Figure 17 displays the reverse bias voltage profile of the specific detectivity (\({D}^{*}\)) of the n-TPA-IFA/p-Si heterostructure. The change of \({D}^{*}\) depending on the light power (\(P\)) applied at − 3 V is given in the inset of Fig. 17. By increasing the light intensity from 20 to 100 mW/cm2, the computed values of \({D}^{*}\) at − 3 V decreased from 3.22 × 109 Jones to 1.75 × 109 Jones. Figure 17 and the inset of Fig. 17 show how the \({D}^{*}\) varies when the reverse bias voltage and light intensity change. Also, \({D}^{*}\) values depending on light intensity are also given in Table 3.
As shown in Fig. 18, the produced n-TPA-IFA/p-Si photodetector's predicted LDR values increase from 10.15 to 18.84 dB when light intensity is raised from 20 to 100 mW/cm2. LDR is essential because image sensors must function over various intensities. For example, if the LDR is sufficiently wide, a clear picture can be created in any situation [72, 73]. LDR values rise as the light intensity rises. Considering the findings, the n-TPA-IFA/p-Si heterojunction diode can be used as an image sensors and photo-devices. LDR values depending on light intensity are also given in Table 3. In the literature, many researchers determined the photodetector parameters with the photodiode they produced [74,75,76]. For example, Yildirim et al. have fabricated the Al/CoPOM/p-Si diode. They determined various photodetector parameters of the diode; it reportedly demonstrated photovoltaic property with a maximum photoresponsivity (R) of 5.13 A/W, photosensitivity (PS(%)) of 2.28, and specific detectivity (D*) of 1.91 × 1010 Jones under 100 mW/cm2 [74]. Chaudhary et al. deposited the BODIPY interfacial layer on n-type Si by spin coating method and prepared the n-Si/BODIPY/Ag diode [75]. They examined the photodetector parameters of the produced diode at various illumination intensities. They calculated the R, (PS(%)), and D* parameter values as 14.51 A/W, 1.06, and 7.9 × 1012 Jones at 100 mW/cm2, respectively.
4 Conclusions
In conclusion, we report for the first time n-type TPA-IFA (“D-A-π-A”) compound, in which triphenylamine is used as a donor, C = N imine part is an acceptor, benzene is a π-spacer, and ester is an acceptor/anchor. Also, the HOMO and LUMO energies of the TPA-IFA were calculated by DFT/B3LYP/6-311G(d,p) method using Gaussian 09 W. The hall measurements showed that the TPA-IFA sample shows n-type behavior at room temperature. Then, the spin coating method was used to create a heterojunction device based on an n-type TPA-IFA thin film on the p-type Si substrate. We investigated the photodiode and photodetector parameters of the n-TPA-IFA/p-Si heterojunction diode in both dark and different light conditions, considering its possible optoelectronic applications.
The n-TPA-IFA/p-Si heterojunction diode's ideality factor and Schottky barrier height were found to be, respectively, 0.716 eV and 3.01 in the dark and 0.755 eV and 1.63 in the 100 mW/cm2. In this phenomenon, the ideality factor decreases as the Schottky barrier height increases with increasing light intensity. A heterojunction with a high rectification ratio of 104 and a low series resistance of 49 Ω was produced. Its lower series resistance value demonstrates the heterostructure's capability for effective photovoltaic cells. A large rectification ratio is seen for heterostructure contact. A decrease in tunneling resistance and space charge modulation across the interface may be the reason for enhanced electrical properties of heterostructures, such as an increase in barrier height and an ideality factor that deviates from unity.
To further investigate the heterojunction diode's response to light, measurements of its photoresponsivity (\(R\)), photosensitivity (\(PS(\%)\)), specific detectivity (\({D}^{*}\)), and linear dynamic range (LDR) were made. The photodiode's \(R\), \(PS(\%)\), \({D}^{*}\), and LDR values were found to be 11.20 mA/W, 321.76, 3.22 × 109 Jones, and 10.15 dB at − 3 V and 20 mW/cm2, respectively.
According to all the findings, the TPA-IFA organic material is a strong candidate for optoelectronic applications. From the results obtained, it was concluded that the newly synthesized TPA-IFA organic material is a photoactive n-type material and improves device performance, and as a result, it can be used as low-cost optoelectronic devices.
Data availability
The authors of this study declare that the data that support their findings are available within the paper. The datasets that were generated and/or analyzed during the study are also available from the corresponding author upon reasonable request.
References
L. Alibabaei, J.H. Kim, M. Wang, N. Pootrakulchote et al., Molecular design of metal-free D–π-A substituted sensitizers for dye-sensitized solar cells. Energy Environ. Sci. 3(11), 1757–1764 (2010). https://doi.org/10.1039/C0EE00218F
Y.J. Chang, T.J. Chow, Dye-sensitized solar cell utilizing organic dyads containing triarylene conjugates. Tetrahedron 65(24), 4726–4734 (2009). https://doi.org/10.1016/j.tet.2009.04.024
A. Mishra, M.K.R. Fischer, P. Bäuerle, Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48(14), 2474–2499 (2009). https://doi.org/10.1002/anie.200804709
M. Erdoğan, A novel dibenzosuberenone bridged D-A-π-A type dye: photophysical and photovoltaic investigations. J. Mol. Struct. 1232, 130056 (2021). https://doi.org/10.1016/j.molstruc.2021.130056
Y. Li, J. Liu, D. Liu, X. Li et al., D-A-π-A based organic dyes for efficient DSSCs: a theoretical study on the role of π-spacer. Comput. Mater. Sci. 161, 163–176 (2019). https://doi.org/10.1016/j.commatsci.2019.01.033
J. Yang, X. Wang, W.-L. Yim, Q. Wang, Computational study on the intramolecular charge separation of D-A-π-A organic sensitizers with different linker groups. J. Phys. Chem. C 119(47), 26355–26361 (2015). https://doi.org/10.1021/acs.jpcc.5b09117
M.F. Şahin, E. Taşcı, M. Emrullahoğlu, H. Gökce et al., Electrical, photodiode, and DFT studies of newly synthesized π-conjugated BODIPY dye-based Au/BOD-Dim/n-Si device. Physica B 614, 413029 (2021). https://doi.org/10.1016/j.physb.2021.413029
Ş Çavdar, S. Izmirli, H. Koralay, N. Turan et al., Optoelectronic properties of triphenylamine organic thin film layered Al/p-Si /TPA/Al heterojunction for photodiode application. ECS J. Solid State Sci. Technol. 12(4), 045001 (2023). https://doi.org/10.1149/2162-8777/acc68c
B. Topaloğlu Aksoy, G. Keşan, E. Özcan, E. Tanrıverdi Eçik et al., Solution-processable BODIPY decorated triazine photodiodes and their comprehensive photophysical evaluation. New J. Chem. 44(5), 2155–2165 (2020). https://doi.org/10.1039/C9NJ05662A
D. Yang, D. Ma, Development of organic semiconductor photodetectors: from mechanism to applications. Adv. Opt. Mater. 7(1), 1800522 (2019). https://doi.org/10.1002/adom.201800522
S. Demirezen, M. Ulusoy, H. Durmuş, H. Cavusoglu et al., Electrical and photodetector characteristics of schottky structures interlaid with P(EHA) and P(EHA-co-AA) functional polymers by the iCVD method. ACS Omega 8(49), 46499–46512 (2023). https://doi.org/10.1021/acsomega.3c04935
A. Kocyigit, S. Aydogan, Ü. Incekara, M. Yılmaz, The electrical characteristics of the Co/Giemsa/n-Si heterostructure depending on measurement temperatures and frequencies. IEEE Sens. J 23(8), 8184–8191 (2023). https://doi.org/10.1109/JSEN.2023.3255180
N. Kaplan, E. Tasci, M. Emrullahoglu, H. Gökce et al., Analysis of illumination dependent electrical characteristics of α- styryl substituted BODIPY dye-based hybrid heterojunction. J. Mater. Sci. –Mater. Electron. 32(12), 16738–16747 (2021). https://doi.org/10.1007/s10854-021-06231-8
H.H. Gullu, D.E. Yildiz, A. Kocyigit, M. Yildirim, Electrical properties of Al/PCBM:ZnO/p-Si heterojunction for photodiode application. J. Alloys Compd. (2020). https://doi.org/10.1016/j.jallcom.2020.154279
S. Eymur, N. Tugluoglu, A. Apaydin, Ü. Akin et al., Illumination dependent electrical and photovoltaic properties of Au/n-Type Si schottky diode with anthracene-based NAMA interlayer. Ecs J. Solid State Sci. Technol. (2021). https://doi.org/10.1149/2162-8777/abf9ec
S. Özden, N. Avci, O. Pakma, I.A. Kariper, Influence of illumination intensity on the electrical properties of Al/NOA65/p-Si/Al heterojunction MPS device. J. Mater. Sci. –Mater. Electron. 33(16), 12796–12807 (2022). https://doi.org/10.1007/s10854-022-08225-6
K.A. Hussain, G.M. Ali, A. Boubaker, A. Kalboussi, Structural, electrical, and photodetection characterization of a pentacene interdigitated MSM photodiode. J. Electron. Mater. (2023). https://doi.org/10.1007/s11664-023-10826-8
M. Jin, Y. Chen, W. Song, H. Lian et al., Synthesis, characterization, and electroluminescent properties of indazole, pyrazole, and triazole/triphenylamine-based compounds. Dyes Pigm. 173, 106912 (2020). https://doi.org/10.1016/j.dyepig.2018.07.058
T. Keawin, N. Prachumrak, S. Namuangruk, S. Pansay et al., Efficient bifunctional materials based on pyrene- and triphenylamine-functionalized dendrimers for electroluminescent devices. RSC Adv. 5(90), 73481–73489 (2015). https://doi.org/10.1039/C5RA07161E
S.-L. Lai, Q.-X. Tong, M.-Y. Chan, T.-W. Ng et al., Distinct electroluminescent properties of triphenylamine derivatives in blue organic light-emitting devices. J. Mater. Chem. 21(4), 1206–1211 (2011). https://doi.org/10.1039/C0JM02550J
S. Tao, Y. Zhou, C.-S. Lee, S.-T. Lee et al., A triphenylamine derivative as a single-emitting component for highly-efficient white electroluminescent devices. J. Mater. Chem. 18(33), 3981–3984 (2008)
P. Agarwala, D. Kabra, A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): evolution and molecular engineering. J. Mater. Chem. 5(4), 1348–1373 (2017). https://doi.org/10.1039/C6TA08449D
F. Bahrani, R. Hameed, S. Resan, M. Al-Anber, Impact of torsion angles to tune efficient dye-sensitized solar cell/donor-π-acceptor model containing triphenylamine: DFT/TD-DFT study. Acta Phys. Polonica A 141(6), 561–568 (2022). https://doi.org/10.12693/APhysPolA.141.561
S. Bibi, R. Ahmad Khera, A. Farhat, J. Iqbal, Triphenylamine based donor-acceptor-donor type small molecules for organic solar cells. Computational Theor. Chem. 1198, 113176 (2021). https://doi.org/10.1016/j.comptc.2021.113176
G. Deogratias, O.S. Al-Qurashi, N. Wazzan, T. Pogrebnaya et al., Effect of substituent in the acceptor on optical and electronic properties of triphenylamine based dyes: a density functional theory/time-dependent density functional theory investigation. Mater. Sci. Semicond. Process. 150, 106935 (2022). https://doi.org/10.1016/j.mssp.2022.106935
A.E. Bejan, C.P. Constantin, M.D. Damaceanu, Evidence of diimide structure variation on overall performance of electro(fluoro)chromic devices integrating versatile triphenylamine-based polyimides. Mater. Today Chem. 26, 101100 (2022). https://doi.org/10.1016/j.mtchem.2022.101100
M. Erdoğan, Z. Orhan, E. Daş, Synthesis of electron-rich thiophene triphenylamine based organic material for photodiode applications. Opt. Mater. 128, 112446 (2022). https://doi.org/10.1016/j.optmat.2022.112446
T.K. Singh, R.A. Grandy, E.S. Dennis, A.S.B. Schouten et al., Exploring structure-property relationships in a family of ferrocene-containing. Triphenylamine-Based Hybrid Organic Dyes, Appl. Sci. 12(12), 6001 (2022). https://doi.org/10.3390/app12126001
Y.-K. Wang, Z.-C. Yuan, G.-Z. Shi, Y.-X. Li et al., Dopant-free spiro-triphenylamine/fluorene as hole-transporting material for perovskite solar cells with enhanced efficiency and stability. Adv. Funct. Mater. 26(9), 1375–1381 (2016). https://doi.org/10.1002/adfm.201504245
M. Yahya, A. Suvitha, A. Bouziani, Structural, photophysical and optoelectronic activity of triphenylamine-based push–pull chromophores: a theoretical study. Opt. Quantum Electron. 54(12), 816 (2022). https://doi.org/10.1007/s11082-022-04239-w
B. Dutta, S. Halder, Fabrication of schottky barrier diodes utilizing schiff base compounds and metal complexes with schiff base ligands. ChemistrySelect 8(24), e202301586 (2023). https://doi.org/10.1002/slct.202301586
G.D. Sharma, Electrical and photoelectrical properties of schottky barrier devices using the chloro aluminium phthalocyanines. Synth. Met. 74(3), 227–234 (1995). https://doi.org/10.1016/0379-6779(95)03360-V
G. Lai, X.R. Bu, J. Santos, E.A. Mintz, Reinvestigation of the vilsmeier-haack formylation of triphenylamine. Synlett 1997(11), 1275–1276 (1997). https://doi.org/10.1055/s-1997-1024
M.J. Frisch, G.W. Trucks, H.B. Schlegel, and G.E.e.a. Scuseria, Gaussian 16 Rev. C.01. 2016: Wallingford, CT.
E. Talebian, M. Talebian, A general review on the derivation of clausius-mossotti relation. Optik 124(16), 2324–2326 (2013). https://doi.org/10.1016/j.ijleo.2012.06.090
N. Tugluoglu, Ö. Yüksel, H. Safak, S. Karadeniz, The double gaussian distribution of inhomogeneous barrier heights in the organic-on-inorganic schottky devices. Phys. Status Solidi a-Appl. Mater. Sci. 209(11), 2313–2316 (2012). https://doi.org/10.1002/pssa.201228163
A.O. Tezcan, S. Eymur, E. Tasci, M. Emrullahoglu et al., Investigation of electrical and photovoltaic properties of Au/n-Si Schottky diode with BOD-Z-EN interlayer. J. Mater. Sci. –Mater. Electron. 32(9), 12513–12520 (2021). https://doi.org/10.1007/s10854-021-05886-7
S. Çavdar, Y. Sahin, N. Turan, H. Koralay et al., Structural and electrical characterization of Cd-doped ZnO thin films produced on p-type Si substrate by SILAR technique. J. Mater. Sci. –Mater. Electron. (2023). https://doi.org/10.1007/s10854-023-11134-x
O. Ongun, E. Tasci, M. Emrullahoglu, Ü. Akin et al., Fabrication, illumination dependent electrical and photovoltaic properties of Au/BOD-Pyr/n-Si/In schottky diode. J. Mater. Sci. –Mater. Electron. 32(12), 15707–15717 (2021). https://doi.org/10.1007/s10854-021-06122-y
M. Baghbanzadeh, L. Belding, L. Yuan, J. Park et al., Dipole-Induced Rectification Across AgTS/SAM//Ga2O3/EGaIn Junctions. J. Am. Chem. Soc. 141(22), 8969–8980 (2019). https://doi.org/10.1021/jacs.9b02891
L. Belding, S.E. Root, Y. Li, J. Park et al., Conformation, and charge tunneling through molecules in SAMs. J. Am. Chem. Soc. 143(9), 3481–3493 (2021). https://doi.org/10.1021/jacs.0c12571
H. Kang, G.D. Kong, S.E. Byeon, S. Yang et al., Interplay of fermi level pinning, marcus inverted transport, and orbital gating in molecular tunneling junctions. J. Phys. Chem. Lett. 11(20), 8597–8603 (2020). https://doi.org/10.1021/acs.jpclett.0c02509
S.J. Cho, G.D. Kong, S. Park, J. Park et al., Molecularly controlled stark effect induces significant rectification in polycyclic-aromatic-hydrocarbon-terminated n-alkanethiolates. Nano Lett. 19(1), 545–553 (2019). https://doi.org/10.1021/acs.nanolett.8b04488
Y. Li, J. Xie, L. Sun, J. Zeng et al., Monolayer organic crystals for ultrahigh performance molecular diodes. Adv. Sci. 11(10), 2305100 (2024). https://doi.org/10.1002/advs.202305100
A. Barkhordari, H.R. Mashayekhi, P. Amiri, S. Altindal et al., Role of graphene nanoparticles on the electrophysical processes in PVP and PVP:ZnTiO3polymer layers at Schottky diode (SD). Semicond. Sci. Technol. (2023). https://doi.org/10.1088/1361-6641/acd2fa
K. Moraki, S. Bengi, S. Zeyrek, M.M. Bülbül et al., Temperature dependence of characteristic parameters of the Au/C20H12/n-Si Schottky barrier diodes (SBDs) in the wide temperature range. J. Mater. Sci. –Mater. Electron. 28(5), 3987–3996 (2017). https://doi.org/10.1007/s10854-016-6011-2
C.A. Canbay, A. Tataroğlu, A. Dere, A.G. Al-Sehemi et al., Electrical, kinetic and photoelectrical properties of CuAlMnMg shape memory alloy/n-Si Schottky diode. J. Alloy. Compd. 888, 161600 (2021). https://doi.org/10.1016/j.jallcom.2021.161600
A.G. Al-Sehemi, K. Ocakoglu, M. Ince, A. Karabulut et al., The electronic and optoelectronic properties of Al/hydroxymethyl functionalized Zn(II)Pc/p-Si photonic device. Polym. Bull. (2023). https://doi.org/10.1007/s00289-023-04906-2
D. Wood, Optoelectronic Semiconductor Devices. 1994, New York: Prentice Hall.
Ş Karataş, N. Berk, Performance of the illumination dependent electrical and photodiode characteristic of the Al/(GO:PTCDA)/p-Si structures. Opt. Mater. 126, 112231 (2022). https://doi.org/10.1016/j.optmat.2022.112231
R.O. Ocaya, A.G. Al-Sehemi, A. Al-Ghamdi, F. El-Tantawy et al., Organic semiconductor photosensors. J. Alloy. Compd. 702, 520–530 (2017). https://doi.org/10.1016/j.jallcom.2016.12.381
S. Altindal, Y. Azizian-Kalandaragh, M. Ulusoy, G. Pirgholi-Givi, The illumination effects on the current conduction mechanisms of the Au/(Er2O3:PVC)/n-Si (MPS) Schottky diodes. J. Appl. Polym. Sci. (2022). https://doi.org/10.1002/app.52497
E. Özcan, B. Topaloğlu Aksoy, E. Tanrıverdi Eçik, A. Dere et al., Fabrication of hybrid photodiode systems: BODIPY decorated cyclotriphosphazene covalently grafted graphene oxides. Inorg. Chem. Front. 7(16), 2920–2931 (2020). https://doi.org/10.1039/D0QI00468E
H. Seymen, Ş Karataş, Investigation of electrical, photodiode and photovoltaic properties of Au/SiO2/n-Si structures with GO and P3C4MT interface. Mater. Chem. Phys. 310, 128449 (2023). https://doi.org/10.1016/j.matchemphys.2023.128449
A.G. Al-Sehemi, A. Tataroglu, A. Dere, A. Karabulut et al., The structural, electrical, and photoelectrical properties of Al/Cu2CdSnS4 chalcogenide film/p-Si Schottky-type photodiode. J. Mater. Sci. –Mater. Electron. (2023). https://doi.org/10.1007/s10854-023-11465-9
A.A. Noroozi, Y. Abdi, A graphene/Si Schottky diode for the highly sensitive detection of protein. RSC Adv. 9(34), 19613–19619 (2019). https://doi.org/10.1039/C9RA03765A
D.E. Yıldız, A. Kocyigit, M. Yıldırım, Investigation of Ag/ZnO/p-Si heterostructure for diode and photodiode applications in visible spectrum. Phys. Scr. 99(1), 015913 (2024). https://doi.org/10.1088/1402-4896/ad0d6e
S.A. Halim, A.-S. Badran, N. Roushdy, E.M. Ahmed et al., A new hybrid structure based Pyranoquinoline-Pyridine derivative: synthesis, optical properties, theoretical analysis, and photodiode applications. J. Mol. Struct. 1293, 136233 (2023). https://doi.org/10.1016/j.molstruc.2023.136233
A.A.M. Farag, A. Ashery, E.M.A. Ahmed, M.A. Salem, Effect of temperature, illumination and frequency on the electrical characteristics of Cu/p-Si Schottky diode prepared by liquid phase epitaxy. J. Alloy. Compd. 495(1), 116–120 (2010). https://doi.org/10.1016/j.jallcom.2010.01.098
A. Alshahrie, A.A. Al-Ghamdi, M.S. Abdel-wahab, W.E. Mahmoud, The structural and optoelectronic properties of Cu1-xTixO (0≤x≤0.05) diodes prepared via a co-sputtering technique. Micro Nanostructures (2022). https://doi.org/10.1016/j.spmi.2021.107115
M.M. Furchi, D.K. Polyushkin, A. Pospischil, T. Mueller, Mechanisms of photoconductivity in atomically thin MoS2. Nano Lett. 14(11), 6165–6170 (2014). https://doi.org/10.1021/n1502339q
S. Demirezen, H.G. Çetinkaya, M. Kara, F. Yakuphanoğlu et al., Synthesis, electrical and photo-sensing characteristics of the Al/(PCBM/NiO: ZnO)/p-Si nanocomposite structures. Sens. Actuators, A 317, 112449 (2021). https://doi.org/10.1016/j.sna.2020.112449
B.A. Gozeh, A. Karabulut, C.B. Ismael, S.I. Saleh et al., Zn-doped CdO effects on the optical, electrical and photoresponse properties of heterojunctions-based photodiodes. J. Alloy. Compd. 872, 159624 (2021). https://doi.org/10.1016/j.jallcom.2021.159624
A. Karabulut, A. Dere, O. Dayan, A.G. Al-Sehemi et al., Silicon based photodetector with Ru(II) complexes organic interlayer. Mater. Sci. Semicond. Process. 91, 422–430 (2019). https://doi.org/10.1016/j.mssp.2018.11.035
A.G. Imer, Y.S. Ocak, Effect of light intensity and temperature on the current voltage characteristics of Al/SY/p-Si organic-inorganic heterojunction. J. Electron. Mater. 45(10), 5347–5355 (2016). https://doi.org/10.1007/s11664-016-4649-4
A. Tombak, Y.S. Ocak, S. Asubay, T. Kilicoglu et al., Fabrication and electrical properties of an organic–inorganic device based on coumarin 30 dye. Mater. Sci. Semicond. Process. 24, 187–192 (2014). https://doi.org/10.1016/j.mssp.2014.03.004
M. Ashtar, K. Yao, M.A. Marwat, J. Yang et al., High performance self-powered photodetector based on CuBi2O4/MAPbI3 heterostructure. Vacuum 219, 112759 (2024). https://doi.org/10.1016/j.vacuum.2023.112759
S. Demirezen, A. Dere, H.G. Çetinkaya, A.G. Al-Sehemi et al., Hybrid photonic device based on Graphene Oxide (GO) doped P3HT-PCBM/p-Silicon for photonic applications. Phys. Scr. 98(11), 115916 (2023). https://doi.org/10.1088/1402-4896/acfce2
F. Sarcan, U. Doğan, A. Althumali, H.B. Vasili et al., A novel NiO-based p-i-n ultraviolet photodiode. J. Alloy. Compd. 934, 167806 (2023). https://doi.org/10.1016/j.jallcom.2022.167806
T. Ji, Q. Liu, R. Zou, Y. Zhang et al., Enhanced UV-visible light photodetectors with a TiO2/Si heterojunction using band engineering. J. Mater. Chem. C 5(48), 12848–12856 (2017). https://doi.org/10.1039/C7TC04811D
V. Balasubramani, J. Chandrasekaran, V. Manikandan, T.K. Le et al., Upgraded photosensitivity under the influence of Yb doped on V2O5 thin films as an interfacial layer in MIS type Schottky barrier diode as photodiode application. J. Solid State Chem. 301, 122289 (2021). https://doi.org/10.1016/j.jssc.2021.122289
S. Demirezen, A.G. Al-Sehemi, A. Yüzer, M. Ince et al., Electrical characteristics and photosensing properties of Al/symmetrical CuPc/p-Si photodiodes. J. Mater. Sci.: Mater. Electron. 33(26), 21011–21021 (2022). https://doi.org/10.1007/s10854-022-08906-2
H. Ko, S. Park, H.J. Son, D.S. Chung, Wide-linear-dynamic-range polymer photodiode with a new benzo [1,2-b:4,5-b′]dithiophene-copolymer: the role of crystalline orientation. Chem. Mater. 32(7), 3219–3228 (2020). https://doi.org/10.1021/acs.chemmater.0c00347
M. Yıldırım, A. Kocyigit, Y. Torlak, E. Yenel et al., Electrical behaviors of the Co- and Ni-based POMs interlayered schottky photodetector devices. Adv. Mater. Interfaces 9(18), 2102304 (2022). https://doi.org/10.1002/admi.202102304
N. Chaudhary, K. Gill, M. Pahuja, S. Rani et al., Silicon distyryl-BODIPY hybrid photodiode: moving a step ahead from organic interface layer to type II band alignment. J. Alloy. Compd. 978, 173389 (2024). https://doi.org/10.1016/j.jallcom.2023.173389
M.S. Salem, A.R. Wassel, M. Fedawy, A. Shaker et al., Integration of biocompatible coomassie brilliant blue dye on silicon in organic/Inorganic heterojunction for photodetection applications. J. Phys. Chem. Solids 169, 110890 (2022). https://doi.org/10.1016/j.jpcs.2022.110890
Acknowledgements
The authors would like to thank Dr. Sait Malkondu from Giresun University for his help in the synthesis of the TPA-IFA compound. The authors wish to acknowledge the financial support of the Scientific Research Projects Foundation of Gazi University.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was financially supported by the Scientific Research Projects Foundation of Gazi University (BAP- FOA-2022-7389).
Author information
Authors and Affiliations
Contributions
ŞC: Investigation, Methodology, Data Analysis, Review and editing, and Methodology. PO: Conceptualization, Experimentation, Data Analysis, and Writing and editing. SE: Conceptualization, Experimentation, Data Analysis, and Writing and editing. NT: Conceptualization, Experimentation, Data Analysis, and Writing and editing.
Corresponding author
Ethics declarations
Competing interests
Şükrü Çavdar, Pınar Oruç, Serkan Eymur, and Nihat Tuğluoğlu declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Çavdar, Ş., Oruç, P., Eymur, S. et al. Investigation of photosensitive and photodetector characteristics of n-TPA-IFA/p-Si heterojunction structure. J Mater Sci: Mater Electron 35, 1022 (2024). https://doi.org/10.1007/s10854-024-12749-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12749-4