Abstract
In this study, the structural, topographic, and optical properties of iron–aluminum oxide (AlxFe2−xO3, 1 > x > 0) thin film grown by co-sputtering method were investigated. Firstly, AlxFe2−xO3 films grown by magnetron RF–DC co-sputtering method were annealed at 540 °C and then XRD analyses were performed. The absorption measurement, Raman shift, XPS, gas sensor measurements, and SEM images were taken, respectively. The band gap energy of the AlxFe2−xO3 samples was obtained and measured as approximately 2.41 eV, and different peaks at various intensities were seen in the XRD analysis. According to the XRD analysis of the AlxFe2−xO3 semiconductor, the peaks occurred at 33.42°, 41.16°, and 54.43° and the XRD analysis results show that the structure is polycrystalline. As seen in the measurement of the AlxFe2−xO3 gas sensors, acoustic gas sensorsgas sensor, the current value increased with the flow of hydrogen gas. This means that the oxygen that hydrogen detached from the thin film surface contributes to the conductivity of the remaining electrons in the structure, which means that the thin film was an n-type semiconductor. It was seen that the AlxFe2−xO3 gas sensor was very sensitive to light. During the feature measurement, it was seen that the current value measured under the light increases considerably.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the industrial environments, homes, workplaces, since air, water, and soil pollution cause serious health problems, serious researches are carried out on gas sensor technology around the world. The air surrounding us contains pollutants and gases harmful to human health in different amounts. Detection of the presence of these gases is of vital importance in terms of ensuring the safety of living things [1]. Basically, when there is a harmful or toxic gas leak, it is first perceived by people as smell, color, and taste. But if the gas leak is a tasteless, colorless, and odorless, poisonous, flammable, or explosive gas with very low concentration, it is very difficult to detect [2, 3]. Traditional detection methods that produce audible alarm systems to warn people are not very reliable as accurate real-time measurements of target gas concentration are required. It is necessary to detect multiple gases with a fast, accurate, and low-cost sensor system at high or very low temperatures. In studies on this subject, metal oxide gas sensors have been found to be quite suitable for this purpose [4, 5].
Different gas sensor technologies have been used for the detection of different gases for centuries, including metal oxide gas sensors, optical gas sensors, catalytic gas sensors, acoustic gas sensors and electrochemical gas sensors. The performance characteristic of each sensor depends on some sensor characteristics such as sensitivity, selectivity, detection limit, response time and return time [6]. Considerable efforts are being made to develop these features of gas sensors [7]. In various studies, it is seen that the AlFe2O3 structure has a wide application area. Metal oxide semiconductor (MOS) thin films have attracted extraordinary attention owing to their efficiency, low cost, reliability, and easy installation. These advantages are of considerable interest for practical applications in different technological areas such as electronics, optoelectronics, and solar energy conversion. Numerous MOS thin film sensors, e.g., CdO, ZnO, WO3, and SnO2, have been fabricated, where the basic mechanism involves chemical reactions over the MOS surface when the target gas comes into contact with the sensitive layer. In addition, optimizing surface morphology by doping with metal transition can greatly enhanced sensor performance such as sensitivity, selectivity, and stability. The AlFe2O3 gas sensor exhibits a high gas response and fast response in detecting low concentrations of various combustible gases [8, 9].
2 Materials and methods
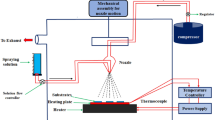
Co-sputter technique is the growth of ions, atoms, and molecules detached from the metal target as a thin layer on the heated substrate with the help of inert gas. In order to detach the material to be grown from the metal target, argon is turned into an inert gas carrier plasma with the help of RF or DC power source and the plasma performs the detachment process by beating the surface of the target metal.
In this experimental study, an iron thin foil target was attached to the DC power supply and an aluminum metal target was attached to the RF power supply part. While the DC power supply is 150 watts, the RF power supply is set to 75 watts. The glass substrates placed for the vacuum chamber up to 7 × 10−6 Torr pressure (base pressure) were heated up to 450 °C [10]. Then 100 sscm of inert argon gas was sent in and plasma was formed. Then, under 8.3 × 10−3 Torr growth pressure, 44 sscm argon and 4 sscm oxygen gas were supplied to the growth chamber and growth was performed for 75 min. Also, the AlxFe2−xO3 films were grown on glass substrates at 8.3 × 10−3 Torr grown pressure.
As can be seen, sputter was made using DC power supply for iron target and RF power supply for aluminum target, and co-sputter was made for AlxFe2−xO3 structure by using both DC and RF power supplies at the same time.
In this study, the structural, optical, and topographic properties of AlxFe2−xO3 structures grown by RF–DC co-sputtering were investigated by Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM), X-ray Diffraction (XRD), Raman Spectroscopy, X-ray Photoelectron Spectroscopy (XPS), and UV–VIS photo spectroscopy [11,12,13,14]. In addition, in this study, the response of AlxFe2−xO3 to hydrogen gas was measured at flow values of 1000 ppm at 300 degrees and for 300, 180, 120 s, respectively. And it has been seen that the examined thin films are suitable for gas sensor application [11].
3 Results and discussion
Firstly, Fig. 1 (left) shows the stretching vibration mode of the Fe2O3 film. Peaks showing the Raman shift are seen. It can be said that these peaks are relatively narrow and intense and show Raman active states belonging to hematite phases at 216, 284, 391, 592, and 1295 cm−1 [15, 16]. It can be said that the shift to large frequency values in the Raman shift of the hematite phase is due to the aluminum doping.
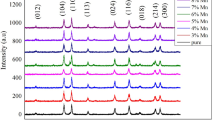
The XRD diffraction pattern of the AlxFe2−xO3 structure grown on the glass substrate is given in Fig. 1 (right) and it has been determined that the structure has an orthorhombic structure. The lattice constants have these values: a = 8.56 A°, b = 9.24 A°, and c = 4.98 A° [Reference code: 00-030-0024]. Three distinct peaks were observed, among which the characteristic AlxFe2−xO3 peaks with orientation at 33.42°, 41.16°, and 54.43°. The values of the three peaks obtained in the XRD analysis results show that there is a polycrystalline structure.
Observing a band gap value different from the pure iron oxide structure, it is seen that (Fig. 2) the aluminum doped affects the band edge. It is also seen from the gas sensor measurements that the structure shows n-type character with the aluminum doped to a material with a p-type character. It is seen that aluminum expands the band gap by affecting the band edge defects [17].
The surface morphology of the AlxFe2−xO3 thin films was analyzed using the non-contact mode of AFM. Figure 3 shows 2D and 3D AFM images of (in Fig. 3 (right)) the AlxFe2−xO3 films grown on glass substrates at 8.3 mTorr grown pressure. Roughness values of film are Ra = 3.78 nm and Sa = 4.34 nm. In the SEM image (Fig. 3 left), it is seen that the structure is homogeneously distributed on the surface. In addition, the lighter colored structures with orthorhombic shapes are observed [13].
When looking at the XPS binding energy change graphs (Fig. 4a, b, c), the change in the binding energy of aluminum, iron, and oxygen elements clearly shows the formation of the aluminum iron oxide structure [18]. Also when looking at graphs (Fig. 4a, b), the binding energy of iron and aluminum elements increased due to the electrons they gave to the oxygen atom. In addition, the binding energy of the oxygen ion is decreased due to the electrons that the oxygen receives. This decrease is seen at two different levels (Fig. 4cOxygen ions on the surface). This shows that bonds are made with both aluminum and iron.
For the AlxFe2−xO3 gas sensor measurements, 500 sscm of dry air was sent to the system where the heated sensor material was located. In order for the I–V graph to reach a more stable state, the system was kept stationary in a dry air environment for approximately 1 h. In the first cycle, after the I–V graph stabilized, the measurement was taken in a dry air environment for 300 s, then 1000 ppm hydrogen gas was given, and the current measurement was taken under the dark condition [19]. In the second cycle, 300 s measurement was taken in dry air environment, then 1000 ppm hydrogen gas was given, and current measurement was taken under the light. As seen in the measurement of the AlxFe2−xO3 gas sensor (Fig. 5; Current–Time), the current value increases with the introduction of hydrogen gas [20]. This means that the oxygen that hydrogen detaches from the thin film surface contributes to the conductivity of the remaining electrons in the structure, which means that the thin film is an n-type semiconductor [5]. The pure oxygen gas sent to the sensor measurement system holds the static charges in the environment and on the film surface at a temperature of about 100 °C (O2 + e−→ O−2).
When the ambient temperature increases further (100 °C < T < 300 °C), the oxygen ions that can bond become ions with a new different charge state by capturing the electrons on the film surface. This new ion will want to adhere more to the film surface. In other words, the oxygen ions in the O2− state are more active in the last state (O2−+ e−→O−+ O−).
At higher temperatures, the oxygen ions attached to the surface are more sensitive to incoming hydrogen gas. Oxygen ions on the surface form H2O with hydrogen gas above 300 °C and leave free electrons at the band gab edge (O-(ads) + 2 H→H2O + e−), while these electrons increase the conductivity in an n-type material. It is seen that (Fig. 5) the AlxFe2−xO3 gas sensor is very sensitive to light. During the feature measurement, it is seen that the current value measured under the light increases considerably.
4 Conclusion
Finally, the structural, optical, and topographic properties of AlxFe2−xO3 structures grown by RF–DC co-sputtering were investigated by SEM, AFM, XRD, Raman spectroscopy, XPS, and UV–VIS photo spectroscopy. In addition, in this study, the response of AlxFe2−xO3 to hydrogen gas was measured, and it has been seen that the examined thin films are suitable for gas sensor application. As seen in the measurement of the AlxFe2−xO3 gas sensor, the current value increases with the flow of hydrogen gas [21]. This means that the oxygen that hydrogen detaches from the thin film surface contributes to the conductivity of the remaining electrons in the structure, which means that the thin film is an n-type semiconductor [22]. At higher temperatures, the oxygen ions attached to the surface are more sensitive to incoming hydrogen gas. Oxygen ions on the surface form H2O with hydrogen gas above 300 °C and leave free electrons at the band gab edge (O−(ads) + 2 H→H2O + e−), while these electrons increase the conductivity in an n-type material. It is seen that (Fig. 5) the AlxFe2−xO3 gas sensor is very sensitive to light. During the feature measurement, it is seen that the current value measured under the light increases considerably. As a result of the excitation of the electrons at the band gab edge with light, the electrons that pass into the conduction band increase the current as a result.
Data availability
The data that support the findings of this study are available from the corresponding author, [Erdal Turgut], upon reasonable request.
References
X. Zhenyuan, P. Maosong, Z. Wenhao, Z. Dongzhi, Hydrogen sulfide gas sensor based on graphitic nitrogen carbide/alpha-iron oxide binary nanostructure. IEEE Sens. J. (2021). https://doi.org/10.1109/JSEN.2020.3035821
W. Jin, W. Chen, Y. Li, C. Zhao, Y. Dai, The effect of surface morphology on the response of Fe2O3-loaded vanadium oxide nanotubes gas sensor. Appl. Surf. Sci. 257(16), 7071–7075 (2011)
G. Jian-Wei, Q. Chen, Developing miniature nano structured semiconductor metal oxide gas sensors. Sens. Mater. 18(4), 183 (2005)
Y. Ma, X.Y. Xie, H.Y. Chen, T.H. Zhang, T.T. Debela, The growth mode of α-Fe2O3 thin films by DC magnetron sputtering. Vacuum 194, 110625 (2021)
S.J. Pearton, F. Ren, Y.L. Wang, B.H. Chu, K.H. Chen, C.Y. Chang, D.P. Norton, Recent advances in wide bandgap semiconductor biological and gas sensors. Prog. Mater. Sci. 55(1), 1–59 (2010)
I. Mitsuhiro, S. Asuka, M. Kaori, L. Baowang, S. Yoshitsugu, A. Takayuki, Effects of sputtering conditions on the activities of high-performance CO2 methanation catalysts prepared by a co-sputtering technique using the polygonal barrel system. Appl. Catal. A, Gen. 597, 117557 (2020)
E. Turgut, Ö. Çoban, S. Sarıtaş, S. Tüzemen, M. Yıldırım, E. Gür, Oxygen partial pressure effects on the RF sputtered p-type NiO hydrogen gas sensors. Appl. Surf. Sci. 435, 880–885 (2018)
S.F. Sı, S.L. Yang, X. Yan, Preparation, characterization and gas responsibilities of Al doped α-Fe2O3. Chem. J. Chinese Univ. 11, 2039 (2007)
T.S. Cho, M.S. Yi, D.Y. Noh, S.J. Doh, J.H. Je, Thickness dependence of the crystallization of α-Fe2O3/α-Al2O3 (0001) thin films grown by sputtering. Solid State Phenom. 124, 1213–1216 (2007)
J.S. Han, T. Bredow, D.E. Davey, A.B. Yu, D.E. Mulcahy, The effect of Al addition on gas sensing properties of Fe2O3-based sensors. Sens. Actuators B 75, 18–23 (2001)
A. Rihab Ben, A. Mejda, M.G. Jorge, L. Ahmed, T. Najoua Kamoun, Physical properties investigation and gas sensing mechanism of Al: Fe2O3 thin films deposited by spray pyrolysis. Superlattices Microstruct. 129, 91–104 (2019)
X. Huajing, F. Jianan, L. Jinyao, A. Rashad, W. Hong, L. Yifan, S. Hua, L. Yuanxun, L. Woon-Ming, M. Nasir, M. Chunhong, J. Xian, Strain-regulated sensing properties of α-Fe2O3 nano-cylinders with atomic carbon layers for ethanol detection. J. Mater. Sci. Technol. 68, 132–139 (2021)
E.T. Paweł, R. Sagayaraj, S. Aravazhi, P. Praveen, G. Chandrasekaran, Comment on structural, morphological and magnetic characters of PVP coated ZnFe2O4 nanoparticles. J. Mater. Sci.: Mater. Electron. 29, 2151 (2018)
T. Qiulin, F. Jiahua, L. Wenyi, X. Jijun, Z. Wendong, Acetone sensing properties of a gas sensor composed of carbon nanotubes doped with iron oxide nanopowder. Sensors 15, 28502–28512 (2015). https://doi.org/10.3390/s151128502
L.V. Gasparov, D.B. Tanner, D.B. Romero, H. Berger, G. Margaritondo, L. Forro, Infrared and Raman studies of the Verwey transition in magnetite. Phys. Rev. B: Condens. Matter Mater. Phys. 62(12), 7939–7944 (2000)
A.M. Jubb, H.C. Allen, Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition applied materials and interface. ACS Appl. Mater. Interfaces 2, 2804–2812 (2010)
S. Saritas, M. Kundakci, O. Coban, S. Tuzemen, M. Yildirim, Ni: Fe2O3, Mg: Fe2O3 and Fe2O3 thin films gas sensor application. Phys. B: Condens. Matter 541, 14–18 (2018)
B. Bhagaban, C. Sudhir, Synthesis and characterization of ZnO nanowires and ZnO–CuO nanoflakes from Sputter-deposited brass (Cu0.65–Zn0.35) film and their application in gas sensing. J. Mater. Sci. Technol. 31, 1069–1078 (2015)
G. Neria, A. Bonavitaa, S. Galvagnoa, P. Sicilianob, S. Caponec, CO and NO2 sensing properties of doped-Fe2O3 thin films prepared by LPD. Sens. Actuators B 82, 40–47 (2002)
L. Ruiwu, Z. Yanwen, S. Maolin, G. Zhen, G. Yuanyuan, Y. Xitao, W. Fayu, D. Wutong, Gas sensing selectivity of oxygen-regulated SnO2 films with different microstructure and texture. J. Mater. Sci. Technol. 35, 2232–2237 (2019)
A. Mirzaei, B. Hashemi, K. Janghorban, α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci: Mater. Electron. 27, 3109–3144 (2016). https://doi.org/10.1007/s10854-015-4200-z
L. Sang Kyoo, H. Seong Hui, H. Sung-Ho, C. Won Mi, K. Soonhyun, P. Hyunwoong, J. Min Gi, Synthesis of Al-doped ZnO nanorods via microemulsion method and their application as a CO gas sensor. J. Mater. Sci. Technol. 31, 639–644 (2015)
Acknowledgements
We would like to thank the Eastern Anatolia High Technology Application and Research Center (DAYTAM) for their support.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
One author contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Erdal Turgut.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Turgut, E. Photosensitive iron–aluminum oxide gas sensor. J Mater Sci: Mater Electron 35, 1068 (2024). https://doi.org/10.1007/s10854-024-12627-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12627-z