Abstract
The chemical bath deposition method was used to prepare Cu-doped Sb2S3 thin films from complexes of Cu and Sb with triethanolamine. The as-deposited films with orange coloration had good adherence to the substrate. After an annealing treatment at 300 °C for 30 min in an Ar atmosphere, the resulting films acquired a brown color and exhibited the orthorhombic phase of Sb2S3 with the preferential growth of [hk0] grains. The Sb2S3 films were formed by rod-shaped nanoparticles, while the insertion of Cu ions in Sb2S3 promoted the formation of ribbon-like structures. The analysis of optical properties indicated the narrowing of bandgap energy from 1.9 to 1.8 eV due to the presence of Cu ions. In addition, the photoconductivity of the films increased from 8.3 × 10–6 to 30.5 × 10–6 Ω−1cm−1, while the photosensitivity factor was enhanced by more than 3 times. The performance of photovoltaic devices based on CdS/Sb2S3 and CdS/Cu:Sb2S3 heterojunctions were investigated. Compared with the device using a Sb2S3 layer, the one using a Cu-doped Sb2S3 layer exhibited an increase in open circuit voltage from 118.2 to 205 mV, short circuit current density from 0.14 to 0.34 mA/cm2 and conversion efficiency from 0.02 to 0.12%. The best performance was obtained by a photovoltaic device with a 500 nm Cu-doped Sb2S3 layer reaching an open circuit voltage of 226 mV, a short circuit current density of 0.94 mA/cm2 and conversion efficiency of 0.32%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Antimony trisulfide (Sb2S3) is a low-toxic semiconductor consisting of chemically interconnected ribbons of (Sb4S6)n parallel to the c-axis that are kept together by weak interactions along the b-axis [1]. This semiconductor has a direct energy band gap of 1.7 eV, optical absorption coefficient greater than 104 cm−1 and p-type electrical conductivity [2,3,4]. These properties have made Sb2S3 a promising candidate for use in applications such as photocatalysis [5, 6], photovoltaics [7, 8], thermoelectric devices [9, 10] and sodium ion batteries [11]. Researchers have reported the synthesis of Sb2S3 thin films by techniques such as successive ionic layer adsorption and reaction (SILAR) [12], electrodeposition [13], spray pyrolysis [14], vacuum thermal evaporation [15] and chemical bath deposition (CBD) [16]. Particularly, CBD is a low-cost and low temperature technique that has been suitably used to prepare homogeneous and good quality films on different types of substrates and large-scale deposition areas. For this reason, a considerable number of reports on the synthesis of Sb2S3 thin films with different Sb and S precursors as well as complexing agents are found in the literature [17,18,19,20,21,22,23,24,25,26,27,28]. Usually, this low temperature deposition technique produces amorphous Sb2S3 films, so an annealing process is needed for them to crystallize in the orthorhombic structure. Mandal and Mondal [17], pioneers in the deposition of Sb2S3 films by CBD, reported that an annealing at 300 °C under N2 atmosphere for 1 h caused a decrease in the optical bandgap from 1.85 to 1.74 eV. A similar approach demonstrated that the electrical resistivity of annealed Sb2S3/glass and Sb2S3/SnO2/glass samples was 4.2 × 108 and 5.3 × 106 Ω cm, respectively [18]. This indicates that the type of substrate has a significant influence on the deposition and final properties of the films. Some authors have used the CBD method to prepare Sb2S3 films doped with different metal ions and studied the modification of their electrical and optical properties. For instance, Ag-doped Sb2S3 films were prepared via a CBD route employing a reaction solution with metal salt precursors and sodium thiosulfate at a temperature of 283 K [29]. As an effect of Ag doping, a bandgap decrease from 2.57 to 2.08 eV was observed. Diliegros-Godines et al. [30] used a citrate-mediated CBD process and found that Ag doping promoted an increase in dark conductivity from 1.84 × 10−9 to 1.33 × 10−8 Ω−1 cm−1, but also a decrease in photosensitivity. The insertion of Sn ions in Sb2S3 films was performed by a CBD process that used ethylenediamine tetraacetic acid (EDTA) as complexing agent [31]. Results indicate that, although resulting films (with Sn content from 1.0 to 5.5 at%) had no significant changes in the optical band gap (1.71–1.72 eV), the electrical resistivity decreased from 2.0 × 105 to 1.4 × 104 Ω cm. Cu doping of Sb2S3 films was also attempted with the EDTA-CBD process [32]. The bandgap values of the resulting films (with Cu-doping concentration from 1.3 to 8.7 at%) were reported in the range of 1.70–1.72 eV, while their electrical resistivity decreased from 2.0 × 105 to 0.2 × 104 Ω cm. However, doping of Sb2S3 films with Cu ions by CBD is challenging as the resulting films exhibit the CuSbS2 phase. Although doping with Cu produces a decrease in the conductivity of a Sb2S3 film, the study of electrical conduction due to the incidence of a light field (known as photoconductivity), which is an important parameter to be investigated for the development of optoelectronic devices, is still required.
Herein, we discuss the influence of Cu doping on the crystalline structure, morphology, and optical properties of Sb2S3 films synthesized by a novel CBD route where triethanolamine was used as complexing agent. The resulting films had a deliberate low Cu concentration to avoid the presence of secondary phases that could deteriorate the photoelectrical properties of Sb2S3. Thus, the substitution of Cu ions at the Sb sites promoted the improvement of photoconductivity and photosensitivity. The performance of photovoltaic (PV) devices based on chemically deposited CdS/Sb2S3 and CdS/Cu:Sb2S3 heterojunctions were also investigated.
2 Experimental
2.1 Materials
Antimony (III) chloride (SbCl3), thioacetamide (TAA, C2H5NS), copper (II) chloride dihydrate (CuCl2·2H2O), zinc acetate dihydrate (Zn(CH3COO)2·2H2O), ethylenediaminetetraacetic acid (EDTA, C10H16N2O8) and Triethanolamine (TEA, C6H15NO3) were purchased from Sigma-Aldrich and used without further modification.
2.2 Synthesis of undoped and Cu-doped Sb2S3 thin films
Undoped and Cu doped Sb2S3 thin films were grown on highly resistive ZnS layers previously deposited on soda-lime glass slides. The reaction solution for the deposition of the ZnS layers was prepared in a beaker according to an earlier study [33]. Under constant stirring, 30 ml of 0.1 M Zn(CH3COO)2, 10 ml of 0.1 M EDTA, 30 ml of 0.6 M TAA and 10 ml of deionized water were mixed. Next, the glass substrates were immersed in the beaker with the reaction solution, whose temperature was maintained at 80 °C in a water bath system. After 3 h, optically transparent films were deposited.
The reaction solution for the deposition of Sb2S3 films was prepared in a beaker, under constant stirring, by dissolving 0.5 g of SbCl3 in a mixture of 1 ml of HCl and 2 ml of ethanol. Next, 3 ml of TEA, 6 ml of 1 M TAA and 86 ml of deionized water were sequentially added. Finally, drops of NH4OH were added to adjust the aqueous solution to pH 10. The ZnS/glass substrates were immersed in the beaker with the reaction solution at a temperature of 80 °C, controlled by a water bath system, for 140 min. The resulting films, with red–orange coloration, were strongly adhered to the substrate. The crystallization process was then carried out by annealing the film at 300 °C for 30 min in an Ar atmosphere. The annealed Sb2S3 film, labelled as SSC0, was observed to adopt the characteristic dark brown coloration.
CuCl2/SbCl3 ratios of 1/24, 2/23, 3/22, and 4/21 were included in reaction solutions for the deposition of Cu-doped Sb2S3 films, which were respectively labeled as SSC1, SSC2, SSC3, and SSC4. In all cases the total weight of metal precursors was kept at 0.5 g. The steps to carry out the deposition and annealing process of each film were the same as those used to prepare the undoped Sb2S3 film. In all cases, dark brown films with good adherence to the substrate were obtained.
2.3 Fabrication of PV devices
Sb2S3/CdS and Cu:Sb2S3/CdS heterojunctions were formed on fluorine doped tin oxide (FTO) coated glasses, with surface resistivity of ~ 7 Ω/sq. CdS films with a thickness of approximately 260 nm were prepared by a CBD process consisting of immersing the FTO/glass substrates in a beaker with a reaction solution at a controlled temperature of 80 °C for 120 min. The reaction solution was prepared under constant stirring by adding 37.1 ml of distilled water, 17.5 ml of 0.1 M cadmium nitrate (Cd(NO3)2*4H2O), 10 ml of 1 M trisodium citrate (C6H5Na3O7*2H2O), 1.4 ml of NH4OH and 3.5 ml of 1 M (CH4N2S). The resulting CdS films were annealed at 300 °C for 30 min in Ar atmosphere to improve their conductivity. The yellowish CdS films, used as window layers in the superstrate configuration, were homogeneous and adherent to the substrate. SSC0 and SSC2 films were then deposited as absorber layers on the CdS films. The device structure was then completed by printing circular conductive graphite contacts, with an area of ~ 0.19 cm2, on the SSC0 and SSC2 layers. An additional annealing treatment at 300 ◦C under Ar atmosphere for 30 min was performed to promote the crystallinity of the absorbing films and improve the adhesion of the graphite contact.
2.4 Characterization
The crystalline structure of the films was analyzed with an X-ray diffractometer Bruker D8 Advance (Cu-Kα radiation, λ = 1.54 Å). The system is equipped with an accessory equivalent to the asymmetric Bragg-Brentano geometry to perform measurements in the grazing angle mode. As a complementary technique, Raman spectroscopy measurements were carried out at room temperature by using a Renishaw inVia system equipped with a 514 nm line Ar ion laser. The surface view and cross-section of the films were captured with a field emission scanning electron microscope (FESEM) JEOL (7800 Prime) in order to study their morphology and determine their thickness. Energy-dispersive spectroscopy (EDS) from an EDAX-Pegasus system with accelerating voltage of 25 kV was used to identify the elemental composition of the films. The optical transmittance and reflectance of the films were performed with a UV–vis spectrometer USB2000 + Ocean Optics in the wavelength range from 250 to 800 nm. In order to determine the conductivity of the Sb2S3 films, electrical measurements were accomplished by a programmable source meter Keithley 2400. This system was also used to measure the I–V response of PV devices under the AM1.5G spectrum of an Oriel LCS-100 solar simulator model 94011A that supplies an irradiance of 100 mW/cm2 (1 sun).
3 Results and discussion
3.1 Growth mechanism
Figure 1 shows the evolution of the reaction solution in the preparation of the sample SSC4. Changes in the coloration indicate the different stages in the formation and growth of particles. As known, the CBD process requires the availability of metal-complexes in order to avoid the premature precipitation of oxides. Thus, the film growth is controlled by a complex-decomposition mechanism that is explained as follows:
The availability of Sb3+ ions in the aqueous media begins with the dissociation of SbCl3 as
while the presence of Cu2+ ions in the aqueous medium can be obtained by the dissociation mechanism,
In presence of TEA molecules at pH 10, complexes with the Sb3+ and Cu2+ ions are formed as follows,
Since the Sb-TEA complexes in aqueous media are colorless, the reaction solution becomes predominantly bluish due to the presence of Cu-TEA complexes, as evidenced in Fig. 1(a). TAA in alkaline solution decomposes into
A greenish solution is observed upon adding TAA (see Fig. 1b), which turns brown when it starts heating to 80 °C, as shown in Fig. 1c. This suggests that in solution Cu ions are available earlier than Sb ions. The brown coloration of the solution is characteristic of the formation of CuS nuclei
After 10 min., the reaction solution has an orange-brown color mixture indicating the formation of the first Sb2S3 nuclei
and later appears completely orange (see Fig. 1d). However, throughout the reaction time some available Sb3+ and Cu2+ ions can also be complexed by the OH– ions supplied by NH4OH, forming metallic hydroxides that participate in the heterogeneous nucleation process:
Therefore, the CuS or Sb2S3 nuclei can be formed through the reactions,
The presence of OH– ions also promote the availability of S2– ions
Therefore, as long as Sb3+ or Cu2+ ions are available to react with S2– ions, Sb2S3 or CuS nuclei participate in the formation of primary particles that collide and react to form aggregates in solution or be adsorbed on the substrate to form the film. All the as-deposited films were orange in color and turned dark brown after being annealed.
3.2 Crystalline structure
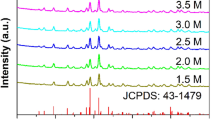
The XRD patterns shown in Fig. 2 were analyzed to determine the crystalline structure of the Sb2S3 films prepared with different Cu concentrations. As seen, the positions of the diffraction peaks of the sample SSC0 are in good agreement with those of the standard (JCPDS Card No. 42-1393) which corresponds to the orthorhombic phase of Sb2S3. Particularly, it is shown that the deposition process favored the preferential growth of [hk0] grains. This effect can be attributed to the constraints of nucleation and growth of particles imposed by the substrate surface. Besides, compared to the standard, the diffraction peaks appear to be slightly shifted towards larger angles due to internal stress. For instance, Table 1 shows the position of the most intense diffraction peak, assigned to the (130) plane. With the insertion of Cu, the diffraction peaks of samples SSC1, SSC2, SSC3, and SSC4 underwent further changes in position towards larger angles which could occur by the difference in ionic radii of Sb3+ (0.76 Å) and Cu2+ (0.71 Å). Moreover, the XRD patterns do not exhibit peaks associated with other crystalline phases. However, the substitution of Cu ions in Sb sites produced crystalline distortions, due to extrinsic defects, which are confirmed by variations in the lattice parameters. Compared to the sample SSC0, the value of lattice parameter b for the Cu-doped Sb2S3 films tends to be lower. In contrast, the values of the lattice parameter a are slightly higher. Basically, it is inferred that the doping process promoted a cation redistribution in the crystalline structure. The average crystallite sizes (D) of the samples, depicted in Table 1, were calculated using the Scherrer equation,
where D is the crystal size, λ is the wavelength of X-ray, β is the FWHM, and θ is the diffraction angle. Results reveal that the Cu doped Sb2S3 films are formed by crystals with D values decreasing from 20 to 18 nm, indicating that the insertion of Cu ions induces disruptions to the crystal growth process. Raman spectroscopy was used to confirm the XRD findings but, given its high sensitivity, also to rule out the presence of secondary crystalline phases. Figure 3 shows the Raman spectra of the undoped and Cu-doped Sb2S3 films. In the Raman spectra of the undoped Sb2S3 film are peaks located at positions that have been associated to the orthorhombic Sb2S3 [34,35,36,37,38]. Specifically, peaks at 139, 191 and 266 and 290 cm−1 are related to Ag modes. Peaks at 114 and 172 cm−1 are associated to B2g vibrational modes and the peak at 223 cm–1 is attributed to the B3g mode. The peaks identified above are also present in the spectra of the Cu-doped Sb2S3 samples with some variations in intensity due to the distortions produced by the insertion of Cu ions in Sb sites. However, the appearance of a peak at 345 cm–1 in the spectrum of the sample SSC4 indicates the emerging formation of CuSbS2 [39].
3.3 Morphology analysis
The surface morphology of the undoped and Cu-doped Sb2S3 thin films were analyzed by SEM. It is known that Sb2S3 particles can grow to adopt different morphologies, including nanotubes and nanorods [40,41,42]. Particularly, the sample SSC0 in Fig. 4(a) is formed by a porous structure of rod-shaped nanoparticles grown parallel to the substrate (see inset) that in most of cases aggregate or coalesce to form flat islands. In good agreement with the XRD results, the presence of planar structures parallel to the substrate correlates with the growth of crystals with preferential orientation (hk0). With the insertion of the Cu precursor, the reaction solution undergoes a supersaturation change that favors the formation of a greater number of nucleation centers. Therefore, there is a greater number of rod-shaped nanoparticles that grow parallel on the lateral face of others, giving rise to the formation of branched planar structures (see Fig. 4b for the sample SSC2). The increase in the concentration of Cu ions in solution leads to the coalescence of many more particles forming ribbon-like structures (see Fig. 4c and d for samples SSC3 and SSC4, respectively). The thickness of the films was measured from cross-sectional images. The samples SSC0, SSC1, SSC2, SSC3, and SSC4 had average thicknesses of 153, 257, 244, 189 and 108 nm, respectively. In congruence with the analysis of surface morphology the nucleation and growth of particles at earlier stages of the reaction promoted the deposition of thicker films. By way of illustration, Fig. 5a and b show cross-sectional images of the samples SSC0 and SSC2, respectively. Unfortunately, the increased nucleation rate due to the increase in the concentrations of Cu ions leads to premature growth of large particles. Some of them precipitate at the bottom of the beaker, while others are deposited on the surface of the substrate. In this way, it is observed that the higher the nucleation speed, the thinner the films. Energy dispersive spectroscopy (EDS) was carried out to identify the presence of the elements in the samples. The EDS spectrum of the sample SSC4 in Fig. 5c shows peaks for Sb and S indicating the presence of Sb2S3. Moreover, there is a weak signal associated with the presence of Cu. Accordingly, the Cu content in samples SSC1, SSC2, SSC3, and SSC4 was found to be 0.04, 0.07, 0.22, and 0.25 at%, respectively. Table 2 depicts the average values semi-quantitative EDS analyses corresponding to Sb, S and Cu content of the samples.
3.4 Optical properties
The optical transmittance (T) spectra of samples SSC0, SSC2, SSC3 and SSC4 are shown in Fig. 6a–d. Aspects such as homogeneity, distribution of surface grains, compactness and thickness are factors that can affect the optical properties of the films. Particularly, the average transmittance of the samples, evaluated in the range from 700 to 1000 nm, exhibit a slight variation between 39.52 and 41.93%. However, the most noticeable effect of the insertion of Cu ions in Sb2S3 is the shift of the absorption edge towards longer wavelengths, indicating a modification of optical band gap which was determined by using the following equation,
where α is the absorption coefficient, hν is the photon energy, A is the constant of proportionality, d is the film thickness of the films and Eg is the optical bandgap. Therefore, the Eg value can be approximated by the intercept of the extrapolated linear portion of (αhv)2 versus hv, as shown by the curve plots in the inset of Fig. 6a–d, for which α was calculated as
The Eg values depicted in Table 3 indicate that the insertion of Cu ions promotes a bandgap narrowing from 1.9 to 1.8 eV which can be ascribed to the emerging of a band formed by the overlap of defect states. Such defect states could originate by structural distortions produced by different accommodations of Cu ions (in the 2+ state) and Sb ions (in the 3+ state) bonded to S ions, and hence the presence of cation or anion vacancies.
3.5 Electrical properties
It has been reported that the inclusion of Cu ions in Sb2S3 films prepared by RF magnetron sputtering promoted a sulfur deficiency, so they presented n-type conductivity [43]. On the contrary, the hot probe test measurements performed on our Cu-doped Sb2S3 films exhibited p-type conductivity, which according to the EDS results can be attributed to rich sulfur content. The electrical conductivity of the films was measured by a pair of graphite electrodes of 5 mm length and a separation of 10 mm printed on the surface. Figure 7 shows the photoresponse plots of the samples as result of measuring the electrical conductivity in dark (σd) and illumination (σl). It is important to note that the conductivity in dark of the Sb2S3 films, whose values are depicted in Table 3, increases monotonically with the concentration of Cu from 2.7 × 10–7 to 9.6 × 10–7 Ω−1cm−1. This is explained below: Substitution of Cu at the Sb site promotes an increase in the density of charge carriers by forming offset defect bands that shift up the valence band [44]. Furthermore, the higher compactness of Cu-doped Sb2S3 films results in better charge transport. Under illumination, electron − hole pairs are generated due to photon absorption which in turn produce a photocurrent (Iph) defined as Iph = Ilight − Idark. After Cu doping, the band gap narrows, and a larger charge carrier density is generated. According to Table 3, the photoconductivity tends to increase until reaching a maximum, as observed in the case of sample SSC2. However, for higher Cu concentrations, more and more charge carriers are generated near the surface region, so they recombine quickly through surface states and do not contribute to the photocurrent. Thus, the decrease in photoconductivity of samples SSC3 and SSC4 reveals that the photocurrent is limited by surface recombination. The photosensitivity (S = (σl – σd)/σd) values listed in Table 3 indicate that the highest S value of sample SSC2 is originated by the highest photocurrent response due a good balance between light absorption enhanced by surface defects and defect-induced carrier traps.
3.6 Performance of PV devices
A PV device (labeled as DSSC2) was fabricated in a glass/FTO/CdS/SSC2/C/Ag configuration, as illustrated in the inset of Fig. 8, to assess the performance of the Cu-doped Sb2S3 film with the better photoresponse. Figure 8 shows the plot of current density versus voltage (J–V) response of such a device, under illumination, compared with that of a device (labelled as DSSC0) fabricated in a glass/FTO/CdS/SSC0/C/Ag configuration. According to Table 4, the open circuit voltage (VOC) and short-circuit current density (JSC) values exhibited by the device DSSC2 were 205 mV and 0.34 mA/cm2, respectively. These values are 73.72% and 150%, respectively, higher than those displayed by the device DSSC0. It has been reported the use of Sb2S3 films prepared by the CBD method for the fabrication of PV devices in the configuration n-Sb2S3/p-Si [45, 46], while other approaches have used post-sulfurized Sb2S3 films in configurations that include electron and hole transport layers [47,48,49,50,51]. However, to the best of our knowledge, there are still no reports on PV devices based on a CdS/Sb2S3 heterojunction. Although the performance of the device DSSC2 is quite low, compared to PV devices that include Sb2S3 films prepared by thermal evaporation [52,53,54], undoubtedly the insertion of Cu ions was a convenient strategy to promote an increase of VOC and JSC. Moreover, the improvement of the fill factor (FF) indicates a slight reduction of series resistance and increase of shunt resistance. Based on the formulation used to prepare the DSSC2 film, six devices with Cu-doped Sb2S3 films were also fabricated by varying the deposition time and number of layers. The device with the best performance, labelled as 2DSSC2 was that processed with a 160 nm CdS film and a 500 nm Cu-doped Sb2S3 film. As shown in Fig. 8, such a device under illumination showed photovoltaic behavior with VOC and JSC values of 226 mV and 0.94 mA/cm2, respectively. Although the conversion efficiency of the device 2DSSC2 (0.32%) is higher than that of the device DSSC2 (0.12%), the decrease in fill factor (FF) from 34 to 30% suggest that further optimizations should be performed by employing metal back contacts instead graphite and buffer or window layers other than CdS [55]. Another challenging strategy is to achieve the chemical deposition of Sb2S3 films with (hk1) orientations in order to get a further improvement in the performance of PV devices, as has been shown with Sb2S3 films deposited by hydrothermal method [56].
4 Conclusions
Successful chemical deposition of Cu-doped Sb2S3 films was demonstrated from the formation of Cu-TEA and Sb-TEA complexes in aqueous solutions. The crystalline structure analysis of the annealed Cu-doped Sb2S3 films indicated the permanence of the orthorhombic phase of Sb2S3. However, the incipient presence of CuSbS2 was detected by Raman spectroscopy in the sample prepared with the highest concentration of Cu doping. Changes in the deposition mechanism due to the presence of Cu ions in the reaction solution were evidenced by observing that the Sb2S3 film was formed of rod-shaped nanoparticles, while the Cu-doped Sb2S3 films were formed of ribbon-like structures. Moreover, the substitution of Cu ions in Sb sites promoted a narrowing of bandgap energy from 2.05 to 1.8 eV, and the improvement in photoconductivity, where the sample SSC2 reached a maximum value of 30.47 × 10–6 Ω−1cm−1. The enhanced photo-generated current due to the presence of Cu ions was also a beneficial strategy to promote a better performance of the PV devices. In summary, the present results support the prospective of the fabricated Cu-doped films to be used as absorbing layers for the development of photosensors and PV devices.
Data availability
Raw data were generated at CINVESTAV-Saltillo. Derived data supporting the findings of this study are available from the corresponding author L. A. G. on request.
References
J. Grigas, Phase transitions in Sb2S3-type chain crystals. Ferroelectrics. 20, 173 (1978). https://doi.org/10.1080/00150197808237202
M.Y. Versavel, J.A. Haber, Structural and optical properties of amorphous and crystalline antimony sulfide thin-films. Thin Solid Films. 515, 7171 (2007). https://doi.org/10.1016/j.tsf.2007.03.043
A.N. Kulkarni, M.B. Rajendra-Prasad, R.V. Ingle, H.M. Pathan, G.E. Eldesoky, M. Naushad, R.S. Patil, Structural and optical properties of nanocrystalline Sb2S3 films deposited by chemical solution deposition. Opt. Mater. 46, 536 (2015). https://doi.org/10.1016/j.optmat.2015.04.066
B. Krishnan, A. Arato, E. Cardenas, T.K. Das-Roy, G.A. Castillo, On the structure, morphology, and optical properties of chemical bath deposited Sb2S3 thin films. Appl. Surf. Sci. 254, 3200 (2008). https://doi.org/10.1016/j.apsusc.2007.10.098
Z.Y. Zhu, J.Y. Li, W. Li, X.Y. Liu, Y.Y. Dang, T.H. Ma, C.Y. Wang, Simulated-sunlight-driven cr(vi) reduction on a type-II heterostructured Sb2S3/CdS photocatalyst, Environ. Sci. : Nano. 9, 1738 (2022). https://doi.org/10.1039/D2EN00050D
J.G. Hao, H.Q. Chen, L. Xie, Z.X. Li, J.L. Zhang, W.Y. Huang, T. Deng, K.Q. Lu, K. Yang, Rationally designed Sb2S3/PDI composites with enhanced visible light photoactivity. Catal. Commun. 162, 106368 (2022). https://doi.org/10.1016/j.catcom.2021.106368
R. Liu, C. Dong, L. Zhu, J. Chen, J. Huang, W. Cao, X. Zhang, C. Ge, S. Yang, T. Chen, M. Wang, Heteroepitaxial and homoepitaxial nucleation strategies to grow Sb2S3 nanorod arrays and therefrom a derived gain of 7.18%-efficient Sb2(S,Se)3 quasi-nanoarray heterojunction solar cells. Appl. Mater. Today. 27, 101487 (2022). https://doi.org/10.1016/j.apmt.2022.101487
J. Zheng, C. Liu, L. Zhang, Y. Chen, F. Bao, J. Liu, H. Zhu, K. Shen, Y. Mai, Enhanced hydrothermal heterogeneous deposition with surfactant additives for efficient Sb2S3 solar cells. Chem. Eng. J. 446–1, 136474 (2022). https://doi.org/10.1016/j.cej.2022.136474
D. Bao, Q. Sun, L. Huang, J. Chen, J. Tang, D. Zhou, M. Hong, L. Yang, Z.G. Chen, Thermoelectric performance of p-type (Bi,Sb)2Te3 incorporating amorphous Sb2S3 nanospheres. Chem. Eng. J. 430–1, 132738 (2022). https://doi.org/10.1016/j.cej.2021.132738
Z. Cui, K. Bu, Y. Zhuang, M.E. Donnelly, D. Zhang, P. Dalladay-Simpson, R.T. Howie, J. Zhang, X. Lü, Q. Hu, Phase transition mechanism and bandgap engineering of Sb2S3 at gigapascal pressures. Commun. Chem. 4, 125 (2021). https://doi.org/10.1038/s42004-021-00565-4
F. Wu, X. Guo, M. Li, H. Xu, One-step hydrothermal synthesis of Sb2S3/reduced graphene oxide nanocomposites for high-performance sodium ion batteries anode materials. Ceram. Int. 43, 6019 (2017). https://doi.org/10.1016/j.ceramint.2017.01.141
M.P. Deshpande, K. Chauhan, K.N. Patel, P. Rajput, H.R. Bhoi, S.H. Chaki, Study of Sb2S3 thin films deposited by SILAR method. Mater. Res. Express. 5, 056410 (2018). https://doi.org/10.1088/2053-1591/aac4ef
A.G. Wallace, R.P. King, N. Zhelev, A.H. Jaafar, W. Levason, R. Huang, G. Reid, P.N. Bartlett, Anodic Sb2S3 electrodeposition from a single source precursor for resistive random-access memory devices. Electrochim. Acta. 432, 141162 (2022). https://doi.org/10.1016/j.electacta.2022.141162
J.S. Eensalu, K. Tõnsuaadu, I.O. Acik, M. Krunks, Sb2S3 thin films by ultrasonic spray pyrolysis of antimony ethyl xanthate. Mater. Sci. Semicond. Process. 137, 106209 (2022). https://doi.org/10.1016/j.mssp.2021.106209
E. Gnenna, N. Khemiri, M. Kong, M.I. Alonso, M. Kanzari, Effect of vacuum annealing on the properties of one step thermally evaporated Sb2S3 thin films for photovoltaic applications. Eur. Phys. J. Appl. Phys. 96, 20301 (2021). https://doi.org/10.1051/epjap/2021210101
M.T.S. Nair, Y. Peña, J. Campos, V.M. García, P.K. Nair, Chemically deposited Sb2S3 and Sb2S3 - CuS Thin films. J. Electrochem. Soc. 145, 2113 (1998). https://doi.org/10.1149/1.1838605
K.C. Mandal, A. Mondal, A new chemical method for preparing semiconductor grade antimony tri-sulphide thin films. J. Phys. Chem. Solids. 51, 1339 (1990). https://doi.org/10.1016/0022-3697(90)90014-7
O. Savadogo, K.C. Mandal, Studies on new chemically deposited photoconducting antimony trisulphide thin films. Sol Energy Mater. Sol Cells. 26, 117 (1992). https://doi.org/10.1016/0927-0248(92)90131-8
J. Desai, C. Lokhande, Alkaline bath chemical deposition of antimony (III) sulphide thin films. Thin Solid Films. 237, 29 (1994). https://doi.org/10.1016/0040-6090(94)90234-8
I. Grozdanov, M. Ristov, G. Sinadinovski, M. Mitreski, Fabrication of amorphous Sb2S3 films by chemical deposition. J. Non-Crystalline Solids. 175, 77 (1994). https://doi.org/10.1016/0022-3093(94)90317-4
R.S. Mane, B.R. Sankapal, C.D. Lokhande, Non-aqueous chemical bath deposition of Sb2S3 thin films. Thin Solid Films. 353, 29 (1999). https://doi.org/10.1016/S0040-6090(99)00362-4
R. Mane, C. Lokhande, SEM and XRD characterization of nanocrystalline Sb2S3 thin films deposited by chemical bath route. Surf. Coat. Tech. 172, 51 (2003). https://doi.org/10.1016/S0257-8972(03)00316-5
A.M. Salem, M.S. Selim, Structure, and optical properties of chemically deposited Sb2S3 thin films. J. Phys. D: Appl. Phys. 34, 12 (2001). https://doi.org/10.1088/0022-3727/34/1/303
F. Ezema, A. Ekwealor, P. Asogwa, P. Ugwuoke, C. Chigbo, R. Osuji, Optical properties and structural characterizations of Sb2S3 thin films deposited by chemical bath deposition technique. Turk. J. Phys. 31, 205 (2007)
H. Maghraoui-Meherzi, T.B. Nasr, N. Kamoun, M. Dachraoui, Structural, morphology and optical properties of chemically deposited Sb2S3 thin films. Phys. B: Cond Matt. 405, 3101 (2010). https://doi.org/10.1016/j.physb.2010.04.020
R. Parize, T. Cossuet, O. Chaix-Pluchery, H. Roussel, E. Appert, V. Consonni, In situ analysis of the crystallization process of Sb2S3 thin films by Raman scattering and X-ray diffraction. Mater. Des. 121, 1 (2017). https://doi.org/10.1016/j.matdes.2017.02.034
S. Shaji, L.V. Garcia, S.L. Loredo, B. Krishnan, J.A. Aguilar-Martinez, T.K. Das-Roy, D.A. Avellaneda, Antimony sulfide thin films prepared by laser assisted chemical bath deposition. Appl. Surf. Sci. 393, 369 (2017). https://doi.org/10.1016/j.apsusc.2016.10.051
U. Chalapathi, B. Poornaprakash, C.H. Ahn, S.H. Park, Rapid growth of Sb2S3 thin films by chemical bath deposition with ethylenediamine tetraacetic acid additive. Appl. Surf. Sci. 451, 272 (2018). https://doi.org/10.1016/j.apsusc.2018.04.249
S.M. Salim, M. Seddek, I.A. Salem, Low-temperature synthesis of Ag-doped Sb2S3 thin films and its characterization. Appl. Sci. Res. 6, 1352 (2010)
C.J. Diliegros-Godines, J. Santos-Cruz, N.R. Mathews, M. Pal, Effect of Ag doping on structural, optical, and electrical properties of antimony sulfide thin films. J. Mater. Sci. 53, 11562 (2018). https://doi.org/10.1007/s10853-018-2420-3
U. Chalapathi, B. Poornaprakash, C.H. Ahn, S.H. Park, Large-grained Sb2S3 thin films with Sn-doping by chemical bath deposition for planar heterojunction solar cells. Mater. Sci. Semicond. Proc. (2018). https://doi.org/10.1016/j.mssp.2018.05.017
U. Chalapathi, B. Poornaprakash, S.H. Park, The effect of Cu-doping on the structural, microstructural, optical, and electrical properties of Sb2S3 thin films. Chalcogen Lett. 16, 449 (2019)
E.G. Alvarez-Coronado, L.A. González, J.C. Rendón-Ángeles, M.A. Meléndez-Lira, R. Ramírez-Bon, Mater. Sci. Semicond. Proc. (2018). https://doi.org/10.1016/j.mssp.2018.03.016
Y. Liu, K.T.E. Chua, T.C. Sum, C.K. Gan, First-principles study of the lattice dynamics of Sb2S3. Phys. Chem. Chem. Phys. 16, 345 (2014). https://doi.org/10.1039/C3CP53879F
J. Ibáñez, J.A. Sans, C. Popescu, J. López-Vidrier, J.J. Elvira-Betanzos, V.P. Cuenca-Gotor, O. Gomis, F.J. Manjón, P. Rodríguez-Hernández, A. Muñoz, Structural, vibrational, and electronic study of Sb2S3 at high pressure. J. Phys. Chem. C 120, 10547 (2016). https://doi.org/10.1021/acs.jpcc.6b01276
I. Watanabe, S. Noguchi, T. Shimizu, Study on local structure in amorphous sb S films by Raman scattering. J. Non-Crystalline Solids. 58, 35 (1983). https://doi.org/10.1016/0022-3093(83)90100-X
M. Medles, N. Benramdane, A. Bouzidi, K. Sahraoui, R. Miloua, R. Desfeux, C. Mathieu, Raman, and optical studies of spray pyrolysed Sb2S3 thin films. J. Optoelectron. Adv. Mater. 16, 726 (2014)
S. Kharbish, S. Jeleň, Raman spectroscopy of the Pb-Sb sulfosalts minerals: Boulangerite, jamesonite, robinsonite and zinkenite. Vib. Spectrosc. 85, 157 (2016). https://doi.org/10.1016/j.vibspec.2016.04.016
V. Vinayakumar, S. Shaji, D.A. Avellaneda, J.A. Aguilar-Martinez, B. Krishnan, Highly oriented CuSbS2 thin films by rapid thermal processing of pre-annealed Sb2S3-Cu layers for PV applications. Mater. Sci. Semicond. Proc. (2019). https://doi.org/10.1016/j.mssp.2018.11.007
X. Shuai, W. Shen, A facile chemical conversion synthesis of Sb2S3 nanotubes and the visible light-driven photocatalytic activities. Nanoscale Res. Lett. 7, 199 (2012). https://doi.org/10.1186/1556-276X-7-199
N. Son, J.Y. Do, M. Kang, Characterization of core@shell-structured ZnO@Sb2S3 particles for effective hydrogen production from water photo spitting. Ceram. Int. 43, 11250 (2017). https://doi.org/10.1016/j.ceramint.2017.05.175
B. Zhou, T. Kimura, Y. Okazaki, K. Hachiya, T. Sagawa, Inheriting Sb2Se3 nanorods on Sb2S3 nanorod arrays for effective light harvesting and charge extraction in solar cells, ACS Appl. Nano Mater. 5, 16082 (2022). https://doi.org/10.1021/acsanm.2c02661
H. Lei, T. Lin, X. Wang, P. Dai, Y. Guo, Y. Gao, D. Hou, J. Chen, Z. Tan, Copper doping of Sb2S3: fabrication, properties, and photovoltaic application. J. Mater. Sci. : Mater. Electron. 30, 21106 (2019). https://doi.org/10.1007/s10854-019-02481-9
R. Zhao, X. Yang, H. Shi, M.H. Du, Intrinsic and complex defect engineering of quasi-one-dimensional ribbons Sb2S3 for photovoltaics performance. Phys. Rev. Mater. 5, 054605 (2021). https://doi.org/10.1103/PhysRevMaterials.5.054605
O. Savadogo, K.C. Mandal, Low-cost technique for preparing n‐Sb2S3/p‐Si heterojunction solar cells. Appl. Phys. Lett. 63, 228 (1993). https://doi.org/10.1063/1.110349
O. Savadogo, K.C. Mandal, Low cost Schottky barrier solar cells fabricated on CdSe and Sb2S3 films chemically deposited with silicotungstic acid. J. Electrochem. Soc. 141, 2871 (1994). https://doi.org/10.1149/1.2059248
S. Ito, K. Tsujimoto, D.C. Nguyen, K. Manabe, H. Nishino, Doping effects in Sb2S3 absorber for full-inorganic printed solar cells with 5.7% conversion efficiency. Int. J. Hydrog Energy. 38, 16749 (2013). https://doi.org/10.1016/j.ijhydene.2013.02.069
P.P. Boix, Y.H. Lee, F. Fabregat-Santiago, S.H. Im, I. Mora-Sero, J. Bisquert, S. Seok, From flat to nanostructured photovoltaics: balance between thickness of the absorber and charge screening in sensitized solar cells. ACS Nano. 6, 873 (2012). https://doi.org/10.1021/nn204382k
T. Muto, G. Larramona, G. Dennler, Unexpected performances of flat Sb2S3-based hybrid extremely thin absorber solar cells. Appl. Phys. Express. 6, 072301 (2013). https://doi.org/10.7567/APEX.6.072301
Y.C. Choi, D.U. Lee, J.H. Noh, E.K. Kim, S. Seok, Highly improved Sb2S3 sensitized-inorganic–organic heterojunction solar cells and quantification of traps by deep-level transient spectroscopy. Adv. Funct. Mater. 24, 3587 (2014). https://doi.org/10.1002/adfm.201304238
E. Zimmermann, T. Pfadler, J. Kalb, J.A. Dorman, D. Sommer, G. Hahn, J. Weickert, L. Schmidt-Mende, Toward high-efficiency solution-processed planar heterojunction Sb2S3 solar cells. Adv. Sci. 2, 1500059 (2015). https://doi.org/10.1002/advs.201500059
J. Escorcia-García, D. Becerra, M.T.S. Nair, P.K. Nair, Heterojunction CdS/Sb2S3 solar cells using antimony sulfide thin films prepared by thermal evaporation. Thin Solid Films. 569, 28 (2014). https://doi.org/10.1016/j.tsf.2014.08.024
S. Yuan, H. Deng, D. Dong, X. Yang, K. Qiao, C. Hu, H. Song, H. Song, Z. He, J. Tang, Efficient planar antimony sulfide thin film photovoltaics with large grain and preferential growth. Sol Energy Mater. Sol Cells. 157, 887 (2016). https://doi.org/10.1016/j.solmat.2016.07.050
X. Chen, Z. Li, H. Zhu, Y. Wang, B. Liang, J. Chen, Y. Xua, Y. Mai, CdS/Sb2S3 heterojunction thin film solar cells with a thermally evaporated absorber. J. Mater. Chem. C 5, 9421 (2017). https://doi.org/10.1039/C7TC02460F
W. Lin, W.-T. Guo, L. Yao, J. Li, L. Lin, J.-M. Zhang, S. Chen, G. Chen, Zn(o,S) buffer layer for in situ hydrothermal Sb2S3 Planar Solar cells, ACS Appl. Mater. Interfaces. 13, 45726 (2021). https://doi.org/10.1021/acsami.1c12501
X. Jin, Y. Fang, T. Salim, M. Feng, S. Hadke, S.W. Leow, T.C. Sum, L.H. Wong, Situ growth of [hk1]-Oriented Sb2S3 for solution-processed Planar Heterojunction Solar cell with 6.4% efficiency. Adv. Funct. Mater. 30, 2002887 (2020). https://doi.org/10.1002/adfm.202002887
Acknowledgements
The authors would like to thank the valuable technical support of Felipe Marquez and Sergio Rodríguez.
Funding
This work was supported by CONAHCyT México through the graduate fellowship program (Grant number CVU 741738).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Susana Salinas-Beltrán, Juniet Gaitán-Arevalo and Luis A. González. The first draft of the manuscript was written by Susana Salinas-Beltrán and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salinas-Beltrán, S., Gaitán-Arevalo, J.R. & González, L.A. Improvement of the photoelectrical properties of chemical bath-deposited Sb2S3 thin films with low copper doping. J Mater Sci: Mater Electron 35, 458 (2024). https://doi.org/10.1007/s10854-024-12256-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12256-6