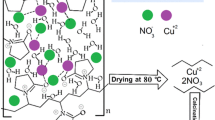
Abstract
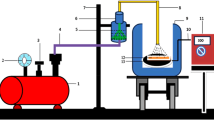
Copper oxide was synthesis by hydrothermal method using copper nano powder (30nm) and deionized water on quartz substrate. At temperature 100 °C, different experimental time ranging from (24–72 h). Different copper oxide phase was presented in X-Ray diffraction analyzer, the crystal size increased as the reaction time increased as it calculated using scherrer equation to be (21.1–32.03 nm), and Williamson Hall plot to be (40.3–87.7 nm). Also, Raman spectroscopy show different copper phase and the highest intensity peak at 220 cm−1, due to the cuprous oxide in all sample used to distinct chemical fingerprint for a particular molecule or material. Many of metal oxide bond was study by FTIR to clarify the compositional and chemical properties of the prepared samples. Optical characteristic with different absorption peaks of thin film show decrease in the band gap from (2.8–2.4 eV) as the reaction time increase. PL measurement used also to determine the band gap energy of the prepared thin film which found to be agreement with UV–Vis, and the band gap value is (2.7, 2.6, 2.5, 2.3, and 2.3 eV), respectively. Increasing in the surface roughness from (3.637–25.16 nm), and in the root mean square from (5–29.39 nm), and grain size with increasing the reaction time from (24–72 h), respectively, was measured by using AFM technique. Different morphologies were showed with increasing the reaction time, spherical and truncated octahedral in different type was obtained by field emission-scanning electron microscope (FE-SEM) as the particles subject to transformation caused the preparing agglomerated crystallites. The electrical characteristic was analysis use DC measurement to measure the activation energy which varied with reaction time. The figure of merit analysis shows that the copper oxidation state to obtain cuprous oxide (Cu2O) is mainly obtained at the 48 h-reaction time. The used of copper nano powder in hydrothermal method without adding any auxiliary material the main aim of this research.














Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study available from the corresponding author “E. T. Salim, “on reasonable request.
References
J.I. Handaker, Hydrothermal synthesis of CuO nanoparticles and a study on property variation with synthesis temperature. J. Appl. Fundam. Sci. 6(2), 52 (2020)
N.M. Shanid, M.A. Khadar, Evolution of nanostructure, phase transition and band gap tailoring in oxidized Cu thin films. Thin Solid Films 516(18), 6245–6252 (2008). https://doi.org/10.1016/j.tsf.2007.11.119
O.A. Abdulrazzaq, E.T. Saleem, Inexpensive near-IR photodetector. Turk. J. Phys. 30, 35–39 (2006)
T.K. Wong et al., Current status and future prospects of copper oxide heterojunction solar cells. Materials 9(4), 271 (2016). https://doi.org/10.3390/ma9040271
F. Oba et al., Epitaxial growth of cuprous oxide electrodeposited onto semiconductor and metal substrates. J. Am. Ceram. Soc. 88(2), 253–270 (2005). https://doi.org/10.1111/j.1551-2916.2005.00118.x
E.T. Salim, Rapid thermal oxidation for silicon nanocrystal based solar cell. Int. J. Nanoelectron. Mater. 5(2), 95–100 (2012)
A.S. Jasim, O.N. Salman, The effect of solvent variation on structural, optical, and electrical properties of TiO2 films prepared by hydrothermal method. J. Appl. Sci. Nanotechnol. 3(2), 59–69 (2023). https://doi.org/10.53293/jasn.2023.5562.1191
Y. Wang, J. Pierson, Binary copper oxides as photovoltaic absorbers: recent progress in materials and applications. J. Phys. D 54(26), 263002 (2021). https://doi.org/10.1088/1361-6463/abf165
M.M. Hassan, M.A. Fakhri, S.A. Adnan, Structural electrical and detection properties of copper oxide based on optoelectronic device. IOP Conf. Ser. 454(1), 012172 (2018). https://doi.org/10.1088/1757-899X/454/1/012172
C. Qin et al., Fabricating high-quality Cu2O photocathode by magnetron sputtering: insight into defect states and charge carrier collection in Cu2O. ACS Appl. Energy Mater. 5(11), 14410–14422 (2022). https://doi.org/10.1021/acsaem.2c02974.s001
L. Li et al., One-pot solid-state reaction approach to synthesize Ag-Cu2O/GO ternary nanocomposites with enhanced visible-light-responsive photocatalytic activity. Int. J. Photoenergy (2017). https://doi.org/10.1155/2017/8983717
B.A. Badr, N.H. Numan, F.G. Khalid, M.A. Fakhri, A.W. Abdulwahhab, All optical investigations of copper oxide for detection devices. J. Ovonic Res. 15(1), 53–59 (2019)
N. Kumar et al., Structural and optical properties of sol–gel derived CuO and Cu2O nanoparticles. Mater. Today 41, 237–241 (2021). https://doi.org/10.1016/j.matpr.2020.08.800
J. Ma et al., Visible-light photocatalytic decolorization of Orange II on Cu2O/ZnO nanocomposites. Ceram. Int. 41(2), 2050–2056 (2015). https://doi.org/10.1016/j.mtchem.2023.101513
M.A. Fakhri, A.W. Abdulwahhab, S.M. Kadhim, M.S. Alwazni, S.A. Adnan, Thermal oxidation effects on physical properties of CuO2 thin films for optoelectronic application. Mater. Res. Express 6(2), 026429 (2018)
X. Lan et al., Morphology-controlled hydrothermal synthesis and growth mechanism of microcrystal Cu2O. CrystEngComm 13(2), 633–636 (2010). https://doi.org/10.1039/c0ce00232a
S.M. Badawy, R. El Khashab, A. Nayl, Synthesis, characterization and catalytic activity of Cu/Cu2O nanoparticles prepared in aqueous medium. Bull. Chem. React. Eng. Catal. 10(2), 169 (2015). https://doi.org/10.9767/bcrec.10.2.7984.169-174
M.M. Hassan, M.A. Fakhri, S.A. Adnan, 2-D of nano photonic silicon fabrication for sensing application. Digest J. Nanomater. Biostruct. 14(4), 873–878 (2019)
N. Abid et al., Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: a review. Adv. Coll. Interface. Sci. 300, 102597 (2022). https://doi.org/10.1016/j.cis.2021.102597
M.A. Fakhri, M.M. Hassan, Morphological and structural properties of Cu2O/2-D photonic silicon nano structure for gas sensors. AIP Conf. Proc. 2213(1), 020244 (2020)
M.N.A.K. Alghurabi, R.S. Mahmood, E.T. Salim, S.F.H. Alhasan, F.G. Khalid, Structure, optical, and morphological investigations of nano copper oxide prepared using RPLD at different laser wavelength effects. Mater. Today 42, 2497–2501 (2021)
L. Pan et al., Cu2O film via hydrothermal redox approach: morphology and photocatalytic performance. J. Phys. Chem. C 118(30), 16335–16343 (2014). https://doi.org/10.1021/jp408056k
S. Cao et al., Hydrothermal synthesis, characterization and gas sensing properties of novel Cu2O open hollow nanospheres. Ceram. Int. 43(5), 4721–4724 (2017). https://doi.org/10.1016/j.ceramint.2016.12.131
K. Liu et al., Influence of pH on hydrothermal synthesis of photoactive Cu2O films in an acetate solution. Int. J. Electrochem. Sci. 17(220660), 2 (2022). https://doi.org/10.20964/2022.06.62
M. Claros et al., Hydrothermal synthesis and annealing effect on the properties of gas-sensitive copper oxide nanowires. Chemosensors 10(9), 353 (2022). https://doi.org/10.3390/chemosensors10090353
X. Jiang et al., Structure, optical properties and photocatalysis performance of Cu 2 O microspheres prepared by hydrothermal method. J. Mater. Sci. 27, 8856–8861 (2016). https://doi.org/10.1007/s10854-016-4911-9
Dai, Y., et al. Cu2O Nanocrystals: study on hydrothermal morphology-modulated synthesis and photodegradation of organic pollutants. In: 3rd International Conference on Material, Mechanical and Manufacturing Engineering (IC3ME 2015). 2015. Atlantis Press, Doi:https://doi.org/10.2991/ic3me-15.2015.355.
X. Chen et al., Hydrothermal synthesis of Cu2O with morphology evolution and its effect on visible-light photocatalysis. Mater. Lett. 297, 129921 (2021). https://doi.org/10.1016/j.matlet.2021.129921
G. Aziz, Hydrothermal synthesis of cuprous oxide nanoflowers and characterization of their optical properties. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi 22(2), 397–401 (2018). https://doi.org/10.19113/sdufbed.58150
F.H. Alsultany, S.F.H. Alhasan, E.T. Salim, Seed layer-assisted chemical bath deposition of Cu2O nanoparticles on ITO-coated glass substrates with tunable morphology, crystallinity, and optical properties. J. Inorg. Organomet. Polym. 31, 3749–3759 (2021). https://doi.org/10.1007/s10904-021-02016-y
M.A. Fakhri, M.N.A.K. Alghurabi, F.H. Alsultany, M.H.A. Wahid, Optical and photolumenance studies of deposited CuO2 nano-structures at different laser energies effects. Defect Diffus. Forum 418, 109–118 (2022)
Z.S. Alshaikhli, S.F.H. Alhasan, E.T. Salim, N.A. Parmin, Visible ranges photo detector fabricated based on nano copper oxide deposited by reactive pulsed laser deposition. Defect Diffus. Forum 418, 89–97 (2022)
M.A. Fakhri, S.F.H. Alhasan, M.A. Abduljabbar, Z.S. Alshaikhli, N.A. Parmin, Fabrication of solar cell based on copper oxide nanostructures deposited using reactive pulsed laser deposition. Defect Diffus. Forum 418, 99–107 (2022)
N.R. Dhineshbabu et al., Study of structural and optical properties of cupric oxide nanoparticles. Appl. Nanosci. 6, 933–939 (2016). https://doi.org/10.1007/s13204-015-0499-2
P. Xu et al., Preparation of binder-free CuO/Cu 2 O/Cu composites: a novel electrode material for supercapacitor applications. RSC Adv. 6(34), 28270–28278 (2016). https://doi.org/10.1039/C6RA00004E
A.D. Faisal, W.K. Kalef, E.T. Salim, F.H. Alsultany, Synthesis of CuO/SnO2 NPs on quartz substrate for temperature sensors application. J Ovonic Res. 18(2), 205–212 (2022)
P. Sberna et al., Sputtered cuprous oxide thin films and nitrogen doping by ion implantation. Thin Solid Films 600, 71–75 (2016). https://doi.org/10.1016/j.tsf.2016.01.005
T.H. Tran, V.T. Nguyen, Phase transition of Cu2O to CuO nanocrystals by selective laser heating. Mater. Sci. Semicond. Process. 46, 6–9 (2016). https://doi.org/10.1016/j.mssp.2016.01.021
N.J. Shukur, A.K. Ali, E.T. Salim, M.A. Fakhri, Effect of surface treatment on the performance and characterization of DS solar cell. AIP Conf. Proc. 2400, 030019 (2022). https://doi.org/10.1063/5.0112128
J. Pan, C. Yang, Y. Gao, Investigations of cuprous oxide and cupric oxide thin films by controlling the deposition atmosphere in the reactive sputtering method. Sens. Mater. 5, 8–9 (2016). https://doi.org/10.18494/sam.2016.1298
P. Bindu, S. Thomas, Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 8, 123–134 (2014). https://doi.org/10.1007/s40094-014-0141-9
H.D. Jabbar, M.J. AbdulRazzaq, M.A. Fakhri, Synthesis porous silicon substrates using electrochemical etching method assisted by laser. AIP Conf. Proc. 2660, 020126 (2022). https://doi.org/10.1063/5.0107762
Pancarana, I.D., et al. Characteristics of copper coated multi-walled carbon nanotube using electroless plating process. In: International Conference on Science and Technology (ICST 2018). 2018. Atlantis Press, Doi:https://doi.org/10.2991/icst-18.2018.198
Raship, N., et al. Effect of annealing temperature on the properties of copper oxide films prepared by dip coating technique. In: AIP Conference Proceedings. 2017. AIP Publishing, Doi:https://doi.org/10.1063/1.4968374
A.Y. Kudhur, E.T. Salim, I. Kara, R.O. Mahdi, F.H. Alsultany, Applications of Cu2O nanoparticles prepared via various techniques: a review paper. Int. J. Nanoelectron. Mater. 15, 131–137 (2022)
A. Monshi, M.R. Foroughi, M.R. Monshi, Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2(3), 154–160 (2012). https://doi.org/10.4236/wjnse.2012.23020
A.Y. Kudhur, E.T. Salim, I. Kara, R.O. Mahdi, R.K. Ibrahim, The effect of laser energy on Cu2O nanoparticles formation by liquid-phase pulsed laser ablation. J. Opt. (2023). https://doi.org/10.1007/s12596-023-01319-2
A.Y. Kudhu, E.T. Salim, I. Kara, M.A. Fakhri, R.O. Mahdi, Structural optical and morphological properties of copper oxide nanoparticles ablated using pulsed laser ablation in liquid. J. Opt. (2023). https://doi.org/10.1007/s12596-023-01331-6
E.T. Salim, I.R. Agool, M.A. Muhsien, Construction of SnO2/SiO2/Si heterojunction and its lineup using I-V and C-V measurements. Int. J. Mod. Phys. B 25(29), 3863–3869 (2011). https://doi.org/10.1142/S0217979211102022
H.H. Kadhim, N. Hasan, A.J. Haider, Characterization of superconductor materials doped with nanoparticles on their properties. J. Appl. Sci. Nanotechnol. 2(3), 18–36 (2022). https://doi.org/10.53293/jasn.2022.4435.1109
W. Zhao et al., Electrodeposition of Cu2O films and their photoelectrochemical properties. CrystEngComm 13(8), 2871–2877 (2011). https://doi.org/10.1039/c0ce00829j
Hattab, F., Fakhry, M., Optical and structure properties for nano titanium oxide thin film prepared by PLD, 2012 First National Conference for Engineering Sciences (FNCES 2012). Doi: https://doi.org/10.1109/NCES.2012.6740474.
D. Gupta et al., Facile synthesis of Cu2O and CuO nanoparticles and study of their structural, optical and electronic properties. J. Alloys Compd. 743, 737–745 (2018). https://doi.org/10.1016/j.jallcom.2018.01.181
R.K. Sendi et al., Copper oxide and copper nanoparticles insertion within a PPy matrix for photodetector applications. Opt. Quant. Electron. 55(11), 956 (2023). https://doi.org/10.1007/s11082-023-05226-5
M.A. Muhsien, E.T. Salim, I.R. Agool, Preparation and characterization of (Au/n-Sn O2 /Si O2 /Si/Al) MIS device for optoelectronic application. Int J Opt 756402, 9 (2013). https://doi.org/10.1155/2013/756402
V. Vinila et al., X-ray diffraction analysis of nano crystalline ceramic PbBaTiO 3. Cryst. Struct. Theory Appl. 3(03), 57 (2014). https://doi.org/10.4236/csta.2014.33007
S. Rehman et al., Tuning structural and optical properties of copper oxide nanomaterials by thermal heating and its effect on photocatalytic degradation of congo red dye. Iran. J. Chem. Chem. Eng. 41(5), 1549–1560 (2022). https://doi.org/10.1016/j.clce.2022.100003
T. Evan, Salim, Optoelectronic properties of Fe2O3/Si heterojunction prepared by rapid thermal oxidation method. Indian J. Phys. 87(4), 349–353 (2013). https://doi.org/10.1007/s12648-012-0229-5
H. Irfan, K. Racik, S. Anand, X-ray peak profile analysis of CoAl2O4 nanoparticles by Williamson-Hall and size-strain plot methods. Modern Electron. Mater. 4(1), 31–40 (2018). https://doi.org/10.3897/j.moem.4.1.33272
H. Shashidharagowda, S.N. Mathad, M.B. Abbigeri, Structural, vibrational and magnetic characterization of copper doped CoMn2O4 nano-particles synthesized by chemical route. Sci. Sinter. 53(4), 429–444 (2021). https://doi.org/10.2298/SOS2104429S
E.T. Salim, M.S. Al-Wazny, M.A. Fakhri, Glancing angle reactive pulsed laser deposition (GRPLD) for Bi 2O3/Si heterostructure. Mod. Phys. Lett. B 27(16), 1350122 (2013). https://doi.org/10.1142/S0217984913501224
A. Sharma et al., Tailoring of structural, optical and electrical properties of anatase TiO2 via doping of cobalt and nitrogen ions. J. Mater. Sci. Technol. 111, 287–297 (2022). https://doi.org/10.1016/j.jmst.2021.09.014
E.H. Livingston, The mean and standard deviation: what does it all mean? J. Surg. Res. 119(2), 117–123 (2004). https://doi.org/10.1016/j.jss.2004.02.008
E.T. Salim, M.A. Fakhri, H. Hassen, Metal oxide nanoparticles suspension for optoelectronic devises fabrication. Int. J. Nanoelectron. Mater. 6(2), 121–128 (2013)
M.A. Muhsien, E.T. Salem, I.R. Agool, H.H. Hamdan, Gas sensing of Au/n-SnO2/p-PSi/c-Si heterojunction devices prepared by rapid thermal oxidation. Appl. Nanosci. 4, 719–732 (2014)
M.A.M. Hassan, M.F.H. Al-Kadhemy, E.T. Salem, Effect irradiation time of Gamma ray on MSISM (Au/SnO2/SiO2/Si/Al) devices using theoretical modeling. Int. J. Nanoelectron. Mater. 8(2), 69–82 (2015)
E.T. Salim, Y. Al-Douri, M.S. Al-Wazny, M.A. Fakhri, Optical properties of Cauliflower-like Bi2O3nanostructures by reactive pulsed laser deposition (PLD) technique. Sol. Energy 107, 523–529 (2014). https://doi.org/10.1016/j.solener.2014.05.020
M. Kaur et al., Highly sensitive NO2 sensor based on ZnO nanostructured thin film prepared by SILAR technique. Sens. Actuators, B Chem. 335, 129678 (2021). https://doi.org/10.1016/j.snb.2021.129678
A. Sahai et al., Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl. Surf. Sci. 390, 974–983 (2016). https://doi.org/10.1016/j.apsusc.2016.09.005
M.A. Muhsien, E.T. Salim, Y. Al-Douri, A.F. Sale, I.R. Agool, Synthesis of SnO2 nanostructures employing Nd:YAG laser. Appl. Phys. A 120(2), 725–730 (2015). https://doi.org/10.1007/s00339-015-9249-2
K. Reimann, K. Syassen, Raman scattering and photoluminescence in Cu 2 O under hydrostatic pressure. Phys. Rev. B 39(15), 11113 (1989). https://doi.org/10.1103/physrevb.39.11113
H.Y.H. Chan, C.G. Takoudis, M.J. Weaver, Oxide film formation and oxygen adsorption on copper in aqueous media as probed by surface-enhanced Raman spectroscopy. J. Phys. Chem. B 103(2), 357–365 (1999). https://doi.org/10.1021/jp983787c
M.A. Fakhri, Y. Al-Douri, U. Hashim, E.T. Salim, D. Prakash, K.D. Verma, Optical investigation of nanophotonic lithium niobate-based optical waveguide. Appl. Phys. B 121(1), 107–116 (2015). https://doi.org/10.1007/s00340-015-6206-x
P.K. Pagare, A. Torane, Band gap varied cuprous oxide (Cu 2 O) thin films as a tool for glucose sensing. Microchim. Acta 183, 2983–2989 (2016). https://doi.org/10.1007/s00604-016-1949-6
H.D. Aghdam et al., Ablation time and laser fluence impacts on the composition, morphology and optical properties of copper oxide nanoparticles. Opt. Mater. 91, 433–438 (2019). https://doi.org/10.1016/j.optmat.2019.03.027
Y.N. Jurn, F. Malek, S.A. Mahmood, W.-W. Liu, M.A. Fakhri, M.H. Salih, Modelling and simulation of rectangular bundle of single-walled carbon nanotubes for antenna applications. Key Eng. Mater. 701, 57–66 (2016). https://doi.org/10.4028/www.scientific.net/KEM.701.57
J. Pavelec et al., Perturbation of water structure by water-polymer interactions probed by FTIR and polarized Raman spectroscopy. J. Mol. Liq. 275, 463–473 (2019). https://doi.org/10.1016/j.molliq.2018.11.023
H. Jiang, Z. Zheng, X. Wang, Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vib. Spectrosc. 46(1), 1–7 (2008). https://doi.org/10.1016/j.vibspec.2007.07.002
Z.T. Salim, U. Hashim, M.K.M. Arshad, M.A. Fakhri, Simulation, fabrication and validation of surface acoustic wave layered sensor based on ZnO/IDT/128° YX LiNBO3. Int. J. Appl. Eng. Res. 11(15), 8785–8790 (2016)
Y. Ghayeb, M. Momeni, M. Menati, Reduced graphene oxide/Cu 2 O nanostructure composite films as an effective and stable hydrogen evolution photocathode for water splitting. J. Mater. Sci. 28, 7650–7659 (2017). https://doi.org/10.1007/s10854-017-6458-9
P. Raul et al., CuO nanorods: a potential and efficient adsorbent in water purification. RSC Adv. 4, 40580–40587 (2014). https://doi.org/10.1039/c4ra04619f
M.A. Fakhri, Y. Al-Douri, U. Hashim, Fabricated optical strip waveguide of nanophotonics lithium niobate. IEEE Photonics J. 8(2), 4500410 (2016). https://doi.org/10.1109/JPHOT.2016.2531583
M. Salavati-Niasari, D. Ghanbari, M.R. Loghman-Estarki, Star-shaped PbS nanocrystals prepared by hydrothermal process in the presence of thioglycolic acid. Polyhedron 35(1), 149–153 (2012). https://doi.org/10.1016/j.poly.2012.01.010
Davari, A., et al., Synthesis and characterization of copper oxide nanoparticles using aqueous extract of Iranian violaceae flower. 2021, https://doi.org/10.15673/fst.v15i3.2178
Y.N. Jurn, F. Malek, S.A. Mahmood, W.-W. Liu, E.K. Gbashi, M.A. Fakhri, Important parameters analysis of the single-walled carbon nanotubes composite materials. ARPN J. Eng. Appl. Sci. 11(8), 5108–5113 (2016)
S.S. Sawant, A.D. Bhagwat, C.M. Mahajan, Synthesis of cuprous oxide (Cu2O) nanoparticles: a review. Жypнaл нaнo-тa eлeктpoннoї фiзики (2016). https://doi.org/10.21272/jnep.8(1).01035
Swarnkar, R., S. Singh, and R. Gopal. Synthesis of copper/copper‐oxide nanoparticles: optical and structural characterizations. In: AIP Conference Proceedings. 2009. American Institute of Physics, https://doi.org/10.1063/1.3183432.
M.A. Fakhri, Y. Al-Douri, E.T. Salim, U. Hashim, Y. Yusof, E.B. Choo, Z.T. Salim, Y.N. Jurn, Structural properties and surface morphology analysis of nanophotonic LINBO3. ARPN J. Eng. Appl. Sci. 11(8), 4974–4978 (2016)
S. Fouad et al., ALD of TiO2/ZnO mutilayers towards the understanding of optical properties and polarizability. Opt. Laser Technol. 140, 107035 (2021). https://doi.org/10.1016/j.optlastec.2021.107035
M.A. Fakhri, U. Hashim, E.T. Salim, Z.T. Salim, Preparation and charactrization of photonic LiNbO3generated from mixing of new raw materials using spry pyrolysis method. J. Mater. Sci. 27(12), 13105–13112 (2016). https://doi.org/10.1007/s10854-016-5455-8
Z.T. Salim, U. Hashim, M.K.M.D. Arshad, M.A. Fakhri, E.T. Salim, Zinc oxide flakes-corolla lobes like nano combined structure for SAW applications. Mater. Res. Bull. 86, 215–219 (2017). https://doi.org/10.1016/j.materresbull.2016.11.015
Z.T. Salim, U. Hashim, M.K.M.D. Arshad, M.A. Fakhri, E.T. Salim, Frequency-based detection of female Aedes mosquito using surface acoustic wave technology: early prevention of dengue fever. Microelectron. Eng. 179, 83–90 (2017). https://doi.org/10.1016/j.mee.2017.04.016
A.Y. Fasasi et al., Effect of precursor solvents on the optical properties of copper oxide thin films deposited using spray pyrolysis for optoelectronic applications. Am. J. Mater. Synth. Process 3(2), 12–22 (2018). https://doi.org/10.11648/j.ajmsp.20180302.12
A. Shabaev, A.L. Efros, A. Nozik, Multiexciton generation by a single photon in nanocrystals. Nano Lett. 6(12), 2856–2863 (2006). https://doi.org/10.1021/nl062059v
M.A. Fakhri, E.T. Salim, M.H.A. Wahid, U. Hashim, Z.T. Salim, R.A. Ismail, Synthesis and characterization of nanostructured LiNbO3 films with variation of stirring duration. J. Mater. Sci. 28(16), 11813–11822 (2017). https://doi.org/10.1007/s10854-017-6989-0
F.A. Akgul et al., Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 147(3), 987–995 (2014). https://doi.org/10.1016/j.matchemphys.2014.06.047
Liu, Z., et al. Preparation of Cu2O Particles and their Photocatalytic Properties. In: IOP Conference Series: Earth and Environmental Science. 2020. IOP Publishing, https://doi.org/10.1088/1755-1315/453/1/012084
E.T. Salim, J.A. Saimon, M.K. Abood, M.A. Fakhri, Some physical properties of Nb2O5 thin films prepared using nobic acid based colloidal suspension at room temperature. Mater. Res. Express 4(10), 106407 (2017). https://doi.org/10.1088/2053-1591/aa90a6
H. Shi et al., Controllable synthesis of novel Cu 2 O micro/nano-crystals and their photoluminescence, photocatalytic and field emission properties. CrystEngComm 14(1), 278–285 (2012). https://doi.org/10.1039/c1ce05868a
S. Jana, P.K. Biswas, Optical characterization of in-situ generated Cu2O excitons in solution derived nano-zirconia film matrix. Mater. Lett. 32(4), 263–270 (1997). https://doi.org/10.1016/s0167-577x(97)00044-x
M.A. Fakhri, M. Halim, A. Wahid, B.A. Badr, E.T. Salim, U. Hashim, Z.T. Salim, Enhancement of Lithium Niobate nanophotonic structures via spin-coating technique for optical waveguides application. Eur. Phys. J. Conf. 162(7), 01004 (2017). https://doi.org/10.1051/epjconf/201716201004
F. Plascencia-Hernández et al., Cu2O cubic and polyhedral structures versus commercial powder: shape effect on photocatalytic activity under visible light. J. Saudi Chem. Soc. 23(8), 1016–1023 (2019). https://doi.org/10.1016/j.jscs.2019.05.007
Li, K., et al. Plasmonic Hot Carrier Transfer Modulated Room–Temperature Visible Light Emission of Cu2O–Au Nanowires. In: Journal of Physics: Conference Series. 2020. IOP Publishing, https://doi.org/10.1088/1742-6596/1575/1/012162.
M.A. Fakhri, M. Halim, A. Wahid, S.M. Kadhim, B.A. Badr, E.T. Salim, U. Hashim, Z.T. Salim, The structure and optical properties of Lithium Niobate grown on quartz for photonics application. Eur. Phys. J. Conf. 162, 01005 (2017). https://doi.org/10.1051/epjconf/201716201005
H. Ding et al., Solvent-controlled synthesis of highly luminescent carbon dots with a wide color gamut and narrowed emission peak widths. Small 14(22), 1800612 (2018). https://doi.org/10.1002/smll.201800612
S. Liu, Y. Wang, Application of AFM in microbiology: a review. Scanning 32(2), 61–73 (2010). https://doi.org/10.1002/sca.20173
Y. Al-Douri, M.A. Fakhri, A. Bouhemadou, R. Khenata, M. Ameri, Stirrer time effect on optical properties of nanophotonic LiNbO3. Mater. Chem. Phys. 203, 243–248 (2018). https://doi.org/10.1016/j.matchemphys.2017.10.024
M.A. Fakhri, H.D. Jabbar, M.J. AbdulRazzaq, ...R.K. Ibrahim, R.A. Ismail, Effect of laser fluence on the optoelectronic properties of nanostructured GaN/porous silicon prepared by pulsed laser deposition. Sci. Rep. 13(1), 21007 (2023). https://doi.org/10.1038/s41598-023-47955-3
Y. Al-Douri, M.A. Fakhri, N. Badi, C.H. Voon, Effect of stirring time on the structural parameters of nanophotonic LiNbO3 deposited by spin-coating technique. Optik 156, 886–890 (2018). https://doi.org/10.1016/j.ijleo.2017.12.059
Huang, G., C.-H. Lu, and H.-H. Yang, Magnetic nanomaterials for magnetic bioanalysis, In: Novel nanomaterials for biomedical, environmental and energy applications. 2019, Elsevier. pp. 89–109, https://doi.org/10.1016/b978-0-12-814497-8.00003-5.
S. Choopun et al., Oxygen pressure-tuned epitaxy and optoelectronic properties of laser-deposited ZnO films on sapphire. Appl. Phys. Lett. 75(25), 3947–3949 (1999). https://doi.org/10.1063/1.125503
M.A. Fakhri, Y. Al-Douri, A. Bouhemadou, M. Ameri, Structural and optical properties of nanophotonic LiNbO 3 under stirrer time effect. J. Opt. Commun. 39(3), 297–306 (2017). https://doi.org/10.1515/joc-2016-0159
A.H. Shukur, Structural, photo-functional and semiconductor properties of copper oxide thin films prepared by dc reactive method under various thicknesses. Kufa J. Eng. 9(1), 133–142 (2018). https://doi.org/10.30572/2018/kje/090109
G. Korotcenkov, The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R. Rep. 61(1–6), 1–39 (2008). https://doi.org/10.1016/j.mser.2008.02.001
M.A. Fakhri, N.H. Numan, Q.Q. Mohammed, M.S. Abdulla, O.S. Hassan, S.A. Abduljabar, A.A. Ahmed, Responsivity and Response Time of Nano Silver Oxide on Silicon Heterojunction Detector. Int. J. Nanoelectron. Mater. 11(21), 109–114 (2018)
K. Govender et al., Understanding the factors that govern the deposition and morphology of thin films of ZnO from aqueous solution. J. Mater. Chem. 14(16), 2575–2591 (2004). https://doi.org/10.1039/b404784b
A.A. Virkar et al., Organic semiconductor growth and morphology considerations for organic thin-film transistors. Adv. Mater. 22(34), 3857–3875 (2010). https://doi.org/10.1002/adma.200903193
M.A. Fakhri, N.H. Numan, Q.Q. Mohammed, M.S. Abdulla, O.S. Hassan, S.A. Abduljabar, A.A. Ahmed, Responsivity and response time of nano silver oxide on silicon heterojunction detector. Int. J. Nanoelectron. Mater. 11(21), 65–72 (2018)
J.-Y. Ho, M.H. Huang, Synthesis of submicrometer-sized Cu2O crystals with morphological evolution from cubic to hexapod structures and their comparative photocatalytic activity. J. Phys. Chem. C 113(32), 14159–14164 (2009). https://doi.org/10.1021/jp903928p
B. Li et al., One-step green synthesis of cuprous oxide crystals with truncated octahedra shapes via a high pressure flux approach. J. Solid State Chem. 184(8), 2097–2102 (2011). https://doi.org/10.1016/j.jssc.2011.05.049
M.K. Abood, M.H.A. Wahid, J.A. Saimon, E.T. Salim, Int. J. Nanoelectron. Mater. 11(21), 237–244 (2018)
M.C. Mevada, B. Sengupta, Effect of temperature and precursor concentration on morphology of copper oxide synthesized on glass substrates via hydrothermal method. Int. J. Sci. Technol. Eng 3, 6–11 (2017). https://doi.org/10.1063/1.5010448
J.L. Elechiguerra, J. Reyes-Gasga, M.J. Yacaman, The role of twinning in shape evolution of anisotropic noble metal nanostructures. J. Mater. Chem. 16(40), 3906–3919 (2006). https://doi.org/10.1039/b607128g
M.A. Fakhri, B.A. Bader, F.G. Khalid, N.H. Numan, A.W. Abdulwahhab, U. Hashim, E.T. Salim, M.A. Munshid, Z.T. Salim, Optical and morphological studies of LiNbO3 nano and micro photonic structural. AIP Conf. Proc. 20, 020017 (2018). https://doi.org/10.1063/1.5080830
T.J. Slade et al., Understanding the thermally activated charge transport in NaPb m SbQ m+ 2 (Q= S, Se, Te) thermoelectrics: weak dielectric screening leads to grain boundary dominated charge carrier scattering. Energy Environ. Sci. 13(5), 1509–1518 (2020). https://doi.org/10.1039/d0ee00491j
M.M. Ibrahim, M.A. Hassan, K.I. Hassoon, Characterization of FeS2 thin film prepared by spray pyrolysis method for optoelectronic applications. J. Appl. Sci. Nanotechnol. 2(3), 78–84 (2022). https://doi.org/10.53293/jasn.2022.3961.1115
D.A. Mohammed, A. Kadhim, M.A. Fakhri, The enhancement of the corrosion protection of 304 stainless steel using Al2O3films by PLD method. AIP Conf. Proc. 2045(1), 020014 (2018). https://doi.org/10.1063/1.5080827
M. Gillet et al., The role of surface oxygen vacancies upon WO3 conductivity. Surf. Sci. 532, 519–525 (2003). https://doi.org/10.1016/s0039-6028(03)00477-1
H. Mahamoud et al., Conductivity and dielectric studies on (Na 0· 4 Ag 0· 6) 2 PbP 2 O 7 compound. Bull. Mater. Sci. 34, 1069–1075 (2011). https://doi.org/10.1007/s12034-011-0163-8
H.T. Halboos, E.T. Salim, SD Niobium (2018) Pentoxide nanostructured thin film, optical structural and morphological properties. IOP Conf. Ser. Mater. Sci. Eng. 454(1), 012174 (2018). https://doi.org/10.1088/1757-899X/454/1/012174
M.S. Al-Wazny, E.T. Salim, BA Bader and MA Fakhry (2018) Synthesis of Bi2O3 films, studying their optical, structural, and surface roughness properties. IOP Conf. Seri. Mater. Sci. Eng. 454(1), 012160 (2018). https://doi.org/10.1088/1757-899X/454/1/012160
E.T. Salim, J.A. Saimon, M.K. Abood, M.A. Fakhri, Electrical conductivity inversion for Nb2O5 nanostructure thin films at different temperatures. Mater. Res. Express 6(12), 126459 (2019). https://doi.org/10.1088/2053-1591/ab771c
A. Linsebigler, G. Lu, J.T. Yates Jr., CO chemisorption on TiO2 (110): oxygen vacancy site influence on CO adsorption. J. Chem. Phys. 103(21), 9438–9443 (1995). https://doi.org/10.1063/1.470005
J. Gunjakar et al., Chemical synthesis of spinel nickel ferrite (NiFe2O4) nano-sheets. Appl. Surf. Sci. 254(18), 5844–5848 (2008). https://doi.org/10.1016/j.apsusc.2008.03.065
M.A. Fakhri, E.T. Salim, M.H.A. Wahid, A.W. Abdulwahhab, U. Hashim, Z.T. Salim, Efficiency enhancement of optical strip waveguide by the effect of heat treatment. Optik 180, 768–774 (2019). https://doi.org/10.1016/j.ijleo.2018.12.006
R. Schropp, A. Madan, Properties of conductive zinc oxide films for transparent electrode applications prepared by rf magnetron sputtering. J. Appl. Phys. 66(5), 2027–2031 (1989). https://doi.org/10.1063/1.344341
B.A. Badr, N.H. Numan, F.G. Khalid, M.A. Fakhri, A.W. Abdulwahhab, Effetcts of substrate temperatures on optical properties and constants of ZnO prepared by PLD. J. Ovonic Res. 15(2), 127–133 (2019)
S.M. Taleb, M.A. Fakhri, S.A. Adnan, Physical investigations of nanophotonic LiNbO3 films for photonic applications. J. Ovonic Res. 15(4), 261–269 (2019)
B.A. Badr, Q.Q. Mohammed, N.H. Numan, M.A. Fakhri, A.W. Abdul Wahhab, Substrate temperature effects on optical properties and constants of ZnO. Int. J. Nanoelectron. Mater. 12(3), 283–290 (2019)
Acknowledgements
The authors would like to thank the department of laser engineering and electro- optic /university of Technology for the logistic support this work.
Funding
No fund has been received for this research study.
Author information
Authors and Affiliations
Contributions
ETS, ROM conceived of the presented idea. ETS, ROM supervised the finding of this work. RAA, ETS, ROM discussed the results and contributed equally to the final manuscript. RAA conducted the experiments. RAA, ETS, ROM provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbas, R.A., Salim, E.T. & Mahdi, R.O. Deposition time effect on copper oxide nano structures, an analysis study using chemical method. J Mater Sci: Mater Electron 35, 427 (2024). https://doi.org/10.1007/s10854-024-12143-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12143-0