Abstract
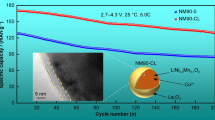
Nickel-rich layered LiNi0.8Co0.15Al0.05O2 (NCA) is considered one of the most promising cathode materials owing to its high energy density and excellent cycling performance. However, the detrimental phase transitions and the interfacial side reactions during cycling have hindered its further development. Here, NCA with different content Li3VO4 coatings (LVO) are synthesized by high temperature solid phase method. The physical and electrochemical performance tests indicate LVO as a fast lithium ion conductor layer effectively improves the Li+ diffusion rate. More importantly, the preparation of fast lithium ion conductor LVO layer consumes residual lithium and prevents the direct contact between the active material and the electrolyte, thus enhancing the stability of electrode material. As a results, the 2.0wt% LVO-NCA shows best electrochemical performance. Even after 300 cycles at 1 C, its capacity retention rate reaches 83.1% compared to 55.3% for pristine NCA. This strategy provides a new idea for the development of high nickel ternary cathode materials.






Similar content being viewed by others
Data availability
All data generated or analyzed for this study are included in this published article [and its supplementary information file].
References
D. Larcher, J.M. Tarascon, Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2014). https://doi.org/10.1038/nchem.2085
W. Li, E.M. Erickson, A. Manthiram, High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy. 5, 26–34 (2020). https://doi.org/10.1038/s41560-019-0513-0
Y. Huang, The discovery of cathode materials for lithium-ion batteries from the view of interdisciplinarity. Interdisciplinary Mater. 1, 323–329 (2022). https://doi.org/10.1002/idm2.12048
M.V. Reddy, G.V.S. Rao, B.V.R. Chowdari, Preparation and characterization of LiNi0.5Co0.5O2 and LiNi0.5Co0.4Al0.1O2 by molten salt synthesis for Li ion batteries. J. Phys. Chem. C 111, 11712–11720 (2007). https://doi.org/10.1021/jp0676890
S. Hu, J. Wang, Y. Lu et al., An epitaxial coating with preferred orientation stabilizing high-energy Ni-Rich NCA cathodes. Appl. Surf. Sci. (2022). https://doi.org/10.1016/j.apsusc.2021.152183
S.-H. Lee, C.S. Yoon, K. Amine et al., Improvement of long-term cycling performance of Li[Ni0.8Co0.15Al0.05]O2 by AlF3 coating. J. Power Sources. 234, 201–207 (2013). https://doi.org/10.1016/j.jpowsour.2013.01.045
J. Yan, H. Hao, J. Tong et al., Recent progress on the modification of high nickel content NCM: coating, doping, and single crystallization. Interdisciplinary Mater. 1, 330–353 (2022). https://doi.org/10.1002/idm2.12043
Y. Kim, H. Park, J.H. Warner et al., Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries. ACS Energy Letters 6, 941–948 (2021). https://doi.org/10.1021/acsenergylett.1c00086
W. Yang, W. Xiang, Y.-X. Chen et al., Interfacial regulation of Ni-Rich cathode materials with an ion-conductive and pillaring layer by infusing gradient boron for improved cycle stability. ACS Appl. Mater. Interfaces 12, 10240–10251 (2020). https://doi.org/10.1021/acsami.9b18542
W.-G. Ryu, H.-S. Shin, M.-S. Park et al., Mitigating storage-induced degradation of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material by surface tuning with phosphate. Ceram. Int. 45, 13942–13950 (2019). https://doi.org/10.1016/j.ceramint.2019.04.092
Y.-Y. Wang, Y.-Y. Sun, S. Liu et al., Na-doped LiNi0.8Co0.15Al0.05O2 with excellent stability of both capacity and potential as cathode materials for Li-Ion batteries. ACS Appl. Energy Mater. 1, 3881–3889 (2018). https://doi.org/10.1021/acsaem.8b00630
Z. Hao, X. Xu, S. Deng et al., In situ growth of Co3O4 coating layer derived from MOFs on LiNi0.8Co0.15Al0.05O2 cathode materials. Ionics. 25, 2469–2476 (2018). https://doi.org/10.1007/s11581-018-2726-9
S. Xia, F. Li, F. Chen et al., Preparation of FePO4 by liquid-phase method and modification on the surface of LiNi0.80Co0.15Al0.05O2 cathode material. J. Alloys Compd. 731, 428–436 (2018). https://doi.org/10.1016/j.jallcom.2017.10.047
H. Li, X. Liu, T. Zhai et al., Li3VO4: a promising insertion anode material for lithium-ion batteries. Adv. Energy Mater. 3, 428–432 (2012). https://doi.org/10.1002/aenm.201200833
Y. Huang, J. Feng-Min et al., Improved cycle stability and high-rate capability of Li3VO4-coated Li[Ni0.5Co0.2Mn0.3]O2 cathode material under different voltages. J. Power Sources. 256, 1–7 (2014). https://doi.org/10.1016/j.jpowsour.2014.01.003
Z. Wang, Z. Wang, H. Guo et al., Improving the cycling stability of LiCoO2 at 4.5V through co-modification by mg doping and zirconium oxyfluoride coating. Ceram. Int. 41, 469–474 (2015). https://doi.org/10.1016/j.ceramint.2014.08.093
D.-C. Li, T. Muta, L.-Q. Zhang et al., Effect of synthesis method on the electrochemical performance of LiNi1/3Mn1/3Co1/3O2. J. Power Sources. 132, 150–155 (2004). https://doi.org/10.1016/j.jpowsour.2004.01.016
X. Lu, X. Li, Z. Wang et al., A modified co-precipitation process to coat LiNi1/3Co1/3Mn1/3O2 onto LiNi0.8Co0.1Mn0.1O2 for improving the electrochemical performance. Appl. Surf. Sci. 297, 182–187 (2014). https://doi.org/10.1016/j.apsusc.2014.01.121
K. Kleiner, D. Dixon, P. Jakes et al., Fatigue of LiNi0.8Co0.15Al0.05O2 in commercial Li ion batteries. J. Power Sources. 273, 70–82 (2015). https://doi.org/10.1016/j.jpowsour.2014.08.133
Z. Wang, H. Zhong, G. Song, Enhancing high-voltage performance of LiNi0.8Co0.1Mn0.1O2 by coating with NASICON fast ionic conductor Li1.5Al0.5Zr1.5(PO4)3. J. Alloys Compd. (2020). https://doi.org/10.1016/j.jallcom.2020.156467
P. He, M. Zhang, J. Wu et al., Enhanced cyclic stability and discharge capacity of NCM811 cathode by coating ferroelectric-type LiNbO3 ionically conductive layer. J. Alloys Compd. (2023). https://doi.org/10.1016/j.jallcom.2023.171822
X. Zheng, X. Li, Z. Wang et al., Investigation and improvement on the electrochemical performance and storage characteristics of LiNiO2-based materials for lithium ion battery. Electrochim. Acta. 191, 32–840 (2016). https://doi.org/10.1016/j.electacta.2016.01.142
H. Yu, Y. Li, Y. Hu et al., 110th anniversary: concurrently coating and doping high-valence vanadium in nickel-rich lithiated oxides for high-rate and stable lithium-ion batteries. Ind. Eng. Chem. Res. 58, 108–4115 (2019). https://doi.org/10.1021/acs.iecr.8b06162
X. Jiang, Y. Wei, X. Yu et al., CeVO4-coated LiNi0.6Co0.2Mn0.2O2 as positive material: towards the excellent electrochemical performance at normal and high temperature. J. Mater. Sci.: Mater. Electron. 29, 5869–15877 (2018). https://doi.org/10.1007/s10854-018-9673-0
H. Zhang, X. Wang, A. Naveed et al., Comparison of structural and electrochemical properties of LiNi0.8Co0.15Al0.05O2 with Li site doping by different cations. Appl. Surf. Sci. (2022). https://doi.org/10.1016/j.apsusc.2022.153933
X. Li, Z. Xie, W. Liu et al., Effects of fluorine doping on structure, surface chemistry, and electrochemical performance of LiNi0.8Co0.15Al0.05O2. Electrochim. Acta. 174, 122–1130 (2015). https://doi.org/10.1016/j.electacta.2015.06.099
M. Chen, Z. Zhang, S. Savilov et al., Enhanced structurally stable cathodes by surface and grain boundary tailoring of Ni-Rich material with molybdenum trioxide. J. Power Sources (2020). https://doi.org/10.1016/j.jpowsour.2020.229051
J.-. Zheng, Z. Yang, Z.-. He et al., In situ formed LiNi0.8Co0.15Al0.05O2@Li4SiO4 composite cathode material with high rate capability and long cycling stability for lithium-ion batteries. Nano Energy. 53, 613–621 (2018). https://doi.org/10.1016/j.nanoen.2018.09.014
H. Qi, K. Liang, W. Guo et al., Facile fabrication and low-cost coating of LiNi0.8Co0.15Al0.05O2 with enhanced electrochemical performance as cathode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 12, 5836–5844 (2017). https://doi.org/10.20964/2017.07.01
M. Seenivasan, C.C. Yang, S.- Wu et al., Improving structural and thermal stability of LiNi0.8Co0.15Al0.05O2 by a fast-ionic-conductive LiAlSiO4 surface coating for Li-ion batteries. Electrochim. Acta (2021). https://doi.org/10.1016/j.electacta.2021.138620
H. Yan, X. Gao, X. Yue et al., Cycling stability of LiNi0.80Co0.15Al0.05O2 cathode modified by solid-state electrolyte film. Appl. Surf. Sci. (2023). https://doi.org/10.1016/j.apsusc.2023.157868
S.N. Lim, W. Ahn, S.-H. Yeon et al., Enhanced elevated-temperature performance of Li(Ni0.8Co0.15Al0.05)O2 electrodes coated with Li2O-2B2O3 glass. Electrochim. Acta. 136, 1–9 (2014). https://doi.org/10.1016/j.electacta.2014.05.056
H. Zhang, X. Zhang, T. Zeng et al., Conversion of residual lithium into fast ionic conductor coating to achieve one-step double modification strategy in LiNi0.8Co0.15Al0.05O2. J. Alloys Compd. (2023). https://doi.org/10.1016/j.jallcom.2022.167638
X. Yang, Y. Tang, G. Shang et al., Enhanced cyclability and high-rate capability of LiNi0.88Co0.095Mn0.025O2 cathodes by homogeneous Al3+ doping. ACS Appl. Mater. Interfaces. 11, 32015–32024 (2019). https://doi.org/10.1021/acsami.9b10558
F. Wu, Q. Li, L. Chen et al., Use of ce to reinforce the interface of Ni-Rich LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium‐ion batteries under high operating voltage. ChemSusChem 12, 935–943 (2019). https://doi.org/10.1002/cssc.201802304
Y. Hou, Y. Ren, T. Shi et al., The surface Al2O3 coating and bulk Zr doping drastically improve the voltage fade and cycling stability of Li(Ni0.8Mn0.1Co0.1)O2 cathode materials. J. Alloys Compd. (2023). https://doi.org/10.1016/j.jallcom.2023.168778
T.-F. Yi, B. Chen, Y.-R. Zhu et al., Enhanced rate performance of molybdenum-doped spinel LiNi0.5Mn1.5O4 cathode materials for lithium ion battery. J. Power Sources. 247, 778–785 (2014). https://doi.org/10.1016/j.jpowsour.2013.09.031
Z. Zhou, Z. Luo, Z. He et al., Suppress voltage decay of lithium-rich materials by coating layers with different crystalline states. J. Energy Chem. 60, 591–598 (2021). https://doi.org/10.1016/j.jechem.2021.01.020
Funding
This work was supported by Open project of Key Laboratory of Catalysis Science and Technology of Chongqing Education Commission (Chongqing Technology and Business University, KFJJ2022012) and Special Key Project of Chongqing Technology Innovation and Application Development (CSTB2023TIAD-KPX0091) and Chongqing National Science Foundation (CSTB2023NSCQ-LZX0039).
Author information
Authors and Affiliations
Contributions
GC: synthesis, characterization, analysis, and manuscript preparation. HY: synthesis and characterization. LL: data curation. ZS: analysis. TY: project administration. MW: methodology. LW: visualization. XH: resources, conceptualization, funding acquisition, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare relevant to this article’s content.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, G., Yang, H., Liu, L. et al. Conversion of residual lithium into fast lithium ion conductor coating to achieve high cycle life LiNi0.8Co0.15Al0.05O2 cathode for lithium ion battery. J Mater Sci: Mater Electron 35, 280 (2024). https://doi.org/10.1007/s10854-024-12063-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12063-z