Abstract
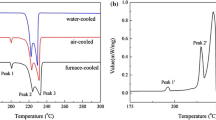
In this work, the corrosion behavior of Sn-3.0Ag-0.5Cu (SAC305) tin-based solder alloy at relatively lower temperature is firstly investigated using electrochemical methods. Effects of temperature and sodium chloride (NaCl) concentration on the corrosion behavior are discussed in detail. Results indicate that the anti-corrosion ability decreased when temperature rising at the same NaCl concentration. On the other hand, the corrosion resistance of SAC305 alloy increased with a lager chloride concentration at relatively lower temperatures, whereas the opposite conclusion was reached at high temperatures. X-ray diffraction meter (XRD) and scanning electron micrograph (SEM) studies revealed that at low temperature, chloride ion reacted with SAC305 alloy forming insoluble basic tin chloride with tin alloy, which covered on metal surface and inhibited corrosion process. However, this process is disrupted at relatively higher temperatures due to chloride ions attacked the tin-oxide protective film on the metal surface, further promoting pitting corrosion. Relevant mechanism has been proposed to clarify the roles of temperature and NaCl concentration in the corrosion process for SAC305 alloy in NaCl solution.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
X. Zhong, W. Lu, B. Liao, B. Medgyes, J. Hu, Y. Zheng, D. Zeng, Z. Zhang, Evidence for ag participating the electrochemical migration of 96.5Sn-3Ag-0.5Cu alloy. Corros. Sci. 156, 10–15 (2019). https://doi.org/10.1016/j.corsci.2019.05.004
M. Fazal, N. Liyana, S. Rubaiee, A. Anas, A critical review on performance, microstructure and corrosion resistance of Pb-free solders. Measurement. 134, 897–907 (2019). https://doi.org/10.1016/j.measurement.2018.12.051
A. Sharma, H.-R. Sohn, J.P. Jung, Effect of Graphene nanoplatelets on Wetting, microstructure, and Tensile characteristics of Sn-3.0Ag-0.5Cu (SAC) Alloy. Metall. Mater. Trans. A 47(1), 494–503 (2016). https://doi.org/10.1007/s11661-015-3214-8
S. Wan, H. Wang, J.H. Liu, B.K. Liao, X.P. Guo, Self-assembled monolayers for electrochemical migration protection of low-temperature sintered nano-Ag paste. Rare Met. 41(4), 1239–1244 (2022). https://doi.org/10.1007/s12598-021-01866-2
C. Nyby, X. Guo, J.E. Saal, S.C. Chien, A.Y. Gerard, H. Ke, T. Li, P. Lu, C. Oberdorfer, S. Sahu, S. Li, C.D. Taylor, W. Windl, J.R. Scully, G.S. Frankel, Electrochemical metrics for corrosion resistant alloys. Sci. Data. 8(1), 58 (2021). https://doi.org/10.1038/s41597-021-00840-y
V. Chidambaram, J. Hald, R. Ambat, J.J.J. Hattel, A corrosion investigation of solder candidates for high-temperature applications. JOM. 61(6), 59–65 (2009). https://doi.org/10.1007/s11837-009-0089-4
X. Zhong, G. Zhang, Y. Qiu, Z. Chen, X. Guo, C. Fu, The corrosion of tin under thin electrolyte layers containing chloride. Corros. Sci. 66, 14–25 (2013). https://doi.org/10.1016/j.corsci.2012.08.040
M. Wang, J. Wang, H. Feng, W. Ke, Effects of microstructure and temperature on corrosion behavior of Sn–3.0 Ag–0.5 cu lead-free solder. J. Mater. Sci. 23(1), 148–155 (2012). https://doi.org/10.1007/s10854-011-0552-1
S. Li, X. Wang, Z. Liu, Y. Jiu, S. Zhang, J. Geng, X. Chen, S. Wu, P. He, W. Long, Corrosion behavior of Sn-based lead-free solder alloys: a review. J. Mater. Sci. 31(12), 9076–9090 (2020). https://doi.org/10.1007/s10854-020-03540-2
B. Illés, H. Choi, T. Hurtony, K. Dušek, D. Bušek, A. Skwarek, Suppression of Sn whisker growth from SnAgCu solder alloy with TiO2 and ZnO reinforcement nano-particles by increasing the corrosion resistance of the composite alloy. J. Mater. Res. Technol. 20, 4231–4240 (2022). https://doi.org/10.1016/j.jmrt.2022.08.172
H.A. Jaffery, M.F.M. Sabri, S.M. Said, S.W. Hasan, I.H. Sajid, N.I.M. Nordin, M.M.I.M. Hasnan, D.A. Shnawah, C.V. Moorthy, Electrochemical corrosion behavior of Sn-0.7 Cu solder alloy with the addition of bismuth and iron. J. Alloys Compd. 810, 151925 (2019). https://doi.org/10.1016/j.jallcom.2019.151925
A. Wierzbicka-Miernik, J. Guspiel, L. Zabdyr, Corrosion behavior of lead-free SAC-type solder alloys in liquid media. Arch. Civ. Mech. Eng 15(1), 206–213 (2015). https://doi.org/10.1016/j.acme.2014.03.003
M. Fayeka, M. Fazal, A. Haseeb, Effect of aluminum addition on the electrochemical corrosion behavior of Sn–3Ag–0.5 Cu solder alloy in 3.5 wt% NaCl solution. J. Mater. Sci. 27(11), 12193–12200 (2016). https://doi.org/10.1007/s10854-016-5374-8
M. Wang, J. Wang, W. Ke, Corrosion behavior of Sn–3.0 Ag–0.5 Cu solder under high-temperature and high-humidity condition. J. Mater. Sci. 25(3), 1228–1236 (2014). https://doi.org/10.1007/s10854-014-1714-8
J.W. Osenbach, J.M. DeLucca, B.D. Potteiger, A. Amin, R.L. Shook, F.A. Baiocchi, Sn Corrosion and its influence on Whisker Growth. IEEE Trans. Electron. Packag. Manuf. 30(1), 23–35 (2007). https://doi.org/10.1109/tepm.2006.890637
D. Zhou, J. Wang, Y. Gao, L. Zhang, Corrosion behavior of tin plate in NaCl solution under different temperature. Int. J. Electrochem. Sci. 12, 192–205 (2017). https://doi.org/10.20964/2017.01.28
C. Qiao, M. Wang, L. Hao, X. Liu, X. Jiang, X. An, D. Li, Temperature and NaCl deposition dependent corrosion of SAC305 solder alloy in simulated marine atmosphere. J. Mater. Sci. Technol. 75, 252–264 (2021). https://doi.org/10.1016/j.jmst.2020.11.012
K. Suganuma, K.-S. Kim, Sn-Zn low temperature solder. J. Mater. Sci. 18, 121–127 (2007). https://doi.org/10.1007/s10854-006-9018-2
G. Chen, X.H. Wang, J. Yang, W.L. Xu, Q. Lin, Effect of micromorphology on corrosion and mechanical properties of SAC305 lead-free solders. Microelectron. Reliab. 108, 113634 (2020). https://doi.org/10.1016/j.microrel.2020.113634
B.K. Liao, Z.X. Liang, Z.G. Luo, Y. Liu, H.W. Deng, T. Zhang, X.P. Guo, Q.S. Ren, H.E. Ge, Insight into microstructure evolution on anti-corrosion property of AlxCoCrFeNiC0.01 high-entropy alloys using scanning vibration electrode technique. Rare Met. 42, 3455–3467 (2023). https://doi.org/10.1007/s12598-023-02322-z
S. Wan, H. Wei, R. Quan, Z. Luo, H. Wang, B. Liao, X. Guo, Soybean extract firstly used as a green corrosion inhibitor with high efficacy and yield for carbon steel in acidic medium. Ind. Crops Prod. 187, 115354 (2022). https://doi.org/10.1016/j.indcrop.2022.115354
B. Liao, Z. Luo, S. Wan, L. Chen, Insight into the anti-corrosion performance of Acanthopanax senticosus leaf extract as eco-friendly corrosion inhibitor for carbon steel in acidic medium. J. Ind. Eng. Chem. 117, 238–246 (2023). https://doi.org/10.1016/j.jiec.2022.10.010
B. Medgyes, G. Kósa, P. Tamási, B. Szabó, B. Illés, M. Lakatos-Varsányi, D. Rigler, L. Gál, M. Ruszinkó, G. Harsányi, Corrosion investigations on lead-free solder alloys in MgCl2 and NaCl solutions, in 2017 IEEE 23rd International Symposium for Design and Technology in Electronic Packaging (SIITME). (IEEE, New York, 2017), pp.427–431. https://doi.org/10.1109/SIITME.2017.8259940
S. Fatimah, Y. Kim, D. Yoon, Y. Ko, Anomaly of corrosion resistance of pure magnesium via soft plasma electrolysis at sub-zero temperature. Surf. Coat. Technol. 385, 125383 (2020). https://doi.org/10.1016/j.surfcoat.2020.125383
M.Z.H. Aziz, N. Zainon, A.A. Mohamad, M.F.M. Nazeri, Corrosion investigation of Sn-0.7Cu Pb-free solder in open-circuit and polarized conditions. IOP Conf. Ser. 957(1), 012012 (2020). https://doi.org/10.1088/1757-899x/957/1/012012
T.-C. Chang, M.-H. Hon, M.-C. Wang, D.-Y. Lin, Electrochemical behaviors of the Sn-9Zn-xAg lead-free solders in a 3.5 wt% NaCl solution. J. Electrochem. Soc. 151(7), C484 (2004). https://doi.org/10.1149/1.1756890
C.W. See, M.Z. Yahaya, H. Haliman, A.A. Mohamad, Corrosion behavior of corroded Sn–3.0 Ag–0.5Cu solder alloy. Procedia Chem. 19, 847–854 (2016). https://doi.org/10.1016/j.proche.2016.03.112
B. Liao, H. Cen, Z. Chen, X. Guo, Corrosion behavior of Sn-3.0Ag-0.5Cu alloy under chlorine-containing thin electrolyte layers. Corros. Sci. 143, 347–361 (2018). https://doi.org/10.1016/j.corsci.2018.08.041
G. Satishkumar, L. Titelman, M. Landau, Mechanism for the formation of tin oxide nanoparticles and nanowires inside the mesopores of SBA-15. J. Solid State Chem. 182(10), 2822–2828 (2009). https://doi.org/10.1016/j.jssc.2009.07.039
C.Q. Cheng, F. Yang, J. Zhao, L.H. Wang, X.G. Li, Leaching of heavy metal elements in solder alloys. Corros. Sci. 53(5), 1738–1747 (2011). https://doi.org/10.1016/j.corsci.2011.01.049
A.F. Mesquita, A.O. Porto, de G.M. Lima, R. Paniago, J.D. Ardisson, The effect of different annealing temperatures on tin and cadmium telluride phases obtained by a modified chemical route. Mater. Res. Bull. 47(11), 3844–3849 (2012). https://doi.org/10.1016/j.materresbull.2012.06.074
J. Donaldson, W. Moser, W. Simpson, 321. Basic tin (II) chloride. J. Chem. Soc. 1963, 1727–1731 (1963)
A. Gharaibeh, I. Felhősi, Z. Keresztes, G. Harsányi, B. Illés, B. Medgyes, Electrochemical corrosion of SAC alloys: a review. Metals 10(10), 1276 (2020). https://doi.org/10.3390/met10101276
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 52001080).
Author information
Authors and Affiliations
Contributions
BKL: Methodology, Writing-Original Draft and Supervision. ZYZ: Experiment design, Experiment and Methodology. ZGL: Writing-Original Draft and Writing-Review. DQW: Visualization, Experiment design and Review.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, BK., Zhou, ZY., Luo, ZG. et al. Insight into influence of temperature on electrochemical corrosion behavior of SAC305 tin-based solder alloy in NaCl solution. J Mater Sci: Mater Electron 34, 2187 (2023). https://doi.org/10.1007/s10854-023-11634-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11634-w