Abstract
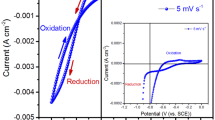
Interconnected nanoflakes of cobalt hydroxide Co(OH)2 were deposited potentiostatically on nickel mesh (NM) at various potentials (− 0.9 V, − 1.0 V, − 1.1 V) and various times (1 min, 2 min, 3 min, 4 min, and 5 min). The FE-SEM, XRD, FT-IR, and EDS etc. studied the morphological and structural properties. The electrochemical properties evaluated in 1 M KOH electrolyte using cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) techniques. The Co(OH)2 nanoflakes deposited on nickel mesh with − 1.0 V for 5 min. electrode exhibits a good specific capacitance of 1035 F g−1, at a current density of 1 mA cm−2 1 M KOH electrolyte. In conclusion, the deposition potential and the deposition time affect the surface morphology and the electrochemical performance of the deposited Co(OH)2 electrode.










Similar content being viewed by others
Data availability
We strive to make our research data accessible to other researchers, subject to any legal, ethical, or privacy restrictions that may apply. We will respond promptly to ensure appropriate access to the data.
References
B. Xu, H. Zhang, H. Mei, D. Sun, Recent progress in metal-organic framework-based supercapacitor electrode materials. Coordin. Chem. Rev. 420, 213438 (2020). https://doi.org/10.1016/j.ccr.2020.213438
F.B. Ajdari, E. Kowsari, M. Niknam Shahrak, A. Ehsani, Z. Kiaei, H. Torkzaban, M. Ershadi, S. Kholghi Eshkalak, V. Haddadi-Asl, A. Chinnappan, S. Ramakrishna, A review on the field patents and recent developments over the application of metal organic frameworks (MOFs) in supercapacitors. Coordin. Chem. Rev. 422, 213441 (2020). https://doi.org/10.1016/j.ccr.2020.213441
L. Yaqoob, T. Noor, N. Iqbal, An overview of supercapacitors electrode materials based on metal organic frameworks and future perspectives. Int. J. Energy Res. 46, 3939–3982 (2021). https://doi.org/10.1002/er.7491
V. Augustyn, P. Simon, B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014)
X. Yi, H. Sun, N. Robertson, C. Kirk, Nanoflower Ni(OH)2 grown in situ on Ni foam for high-performance supercapacitor electrode materials. Sustain. Energy Fuels 5, 5236 (2021). https://doi.org/10.1039/D1SE01036K
S.D. Dhas, P.S. Maldar, M.D. Patil, A.B. Nagare, M.R. Waikar, R.G. Sonkawade, A.V. Moholkar, Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 181, 109646 (2020). https://doi.org/10.1016/j.vacuum.2020.109646
R. Wang, X. Yan, J. Lang, Z. Zhengm, P. Zhang, A hybrid supercapacitor based on flower-like Co(OH)2 and urchin-like VN electrode materials. J. Mater. Chem. A 2, 12724–12732 (2014). https://doi.org/10.1039/C4TA01296H
C. Park, J. Hwang, Y. Hwang, C. Song, S. Ahn, H.S. Kim, H. Ahn, Intense pulsed white light assisted fabrication of Co–CoOx core–shell nanoflakes on graphite felt for flexible hybrid supercapacitors. Electrochim. Acta 246, 757–765 (2017). https://doi.org/10.1016/j.electacta.2017.06.087
T.-F. Yi, J. Mei, B. Guan, P. Cui, S. Luo, Y. Xie, Y. Liu, Construction of spherical NiO@MnO2 with core-shell structure obtained by depositing MnO2 nanoparticles on NiO nanosheets for high-performance supercapacitor. Ceram. Int. 46, 421–429 (2020). https://doi.org/10.1016/j.ceramint.2019.08.278
Q. Zhou, J. Xing, Y. Gao, X. Lv, Y. He, Z. Guo, Y. Li, Ordered assembly of NiCo2O4 multiple hierarchical structures for high-performance pseudocapacitors. ACS Appl. Mater. Interfaces 6, 11394–11402 (2014). https://doi.org/10.1021/am501988s
Z.-S. Wu, D.-W. Wang, W. Ren, J. Zhao, G. Zhou, F. Li, H.-M. Cheng, Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors. Adv. Funct. Mater. 20, 3595–3602 (2010). https://doi.org/10.1002/adfm.201001054
N. Wang, J. He, K. Wang, Y. Zhao, T. Jiu, C. Huang, Y. Li, Graphdiyne-based materials: preparation and application for electrochemical energy storage. Adv. Mater. 31, 1803202 (2019). https://doi.org/10.1002/adma.201803202
L. Miao, Z. Song, D. Zhu, L. Li, L. Gan, M. Liu, Recent advances in carbon-based supercapacitors. Mater. Adv. 1, 945–966 (2020). https://doi.org/10.1039/D0MA00384K
S. Bhattacharya, I. Roy, A. Tice, C. Chapman, R. Udangawa, V. Chakrapani, J.L. Plawsky, R.J. Linhardt, High-conductivity and high-capacitance electrospun fibers for supercapacitor applications. ACS Appl. Mater. Interfaces 12, 19369–19376 (2020). https://doi.org/10.1021/acsami
S.K. Shinde, M.B. Jalak, G.S. Ghodake, N.C. Maile, H.M. Yadav, A.D. Jagadale, D.S. Asif Shahzad, A.A. Lee, V.J. Kadam, D.-Y. Kim. Fulari, Flower-like NiCo2O4/NiCo2S4 electrodes on Ni mesh for higher supercapacitor applications. Ceram. Int. 45, 17192–17203 (2019). https://doi.org/10.1016/j.ceramint.2019.05.274
E.M. Abebe, M. Ujihara, Simultaneous electrodeposition of ternary metal oxide nanocomposites for high-efficiency supercapacitor applications. ACS Omega 7, 17161–17174 (2022). https://doi.org/10.1021/acsomega.2c00826
E. Noormohammadi, F. Poli, C. Durante, M. Lunardon, S. Sanjabi, F. Soavi, Electrodeposition of cobalt–copper oxides decorated with conductive polymer for supercapacitor electrodes with high stability. ChemElectroChem 9(2022), e202200102 (2022). https://doi.org/10.1002/celc.202200102
X. Dai, M. Zhang, J. Li, Effects of electrodeposition time on a manganese dioxide supercapacitor. RSC Adv. 10, 15860 (2020). https://doi.org/10.1039/d0ra01681k
F. Ma, X. Dai, J. Jin, N. Tie, Y. Dai, Hierarchical core-shell hollow CoMoS4@ Ni–Co–S nanotubes hybrid arrays as advanced electrode material for supercapacitors. Electrochim. Acta 331, 135459 (2020)
M. Beidaghi, Y. Gogotsi, Capacitive energy storage in micro-scale devices: recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 7(3), 867–884 (2014)
H. Zhang et al., Growth of manganese oxide nanoflowers on vertically-aligned carbon nanotube arrays for high-rate electrochemical capacitive energy storage. Nano Lett. 8(9), 2664–2668 (2008)
B. Xiao, W. Zhu, Z. Li, J. Zhu, X. Zhu, G. Pezzotti, Tailoring morphology of cobalt–nickel layered double hydroxide via different surfactants for high-performance supercapacitor. R. Soc. Open Sci. 5, 180867 (2018). https://doi.org/10.1098/rsos.180867
N.A. Salleh, S. Kheawhom, A.A. Mohamad, Characterizations of nickel mesh and nickel foam current collectors for supercapacitor application. Arab. J. Chem. 13, 6838–6846 (2020). https://doi.org/10.1016/j.arabjc.2020.06.036
N.C. Maile, S.K. Shinde, R.R. Koli, A.V. Fulari, D.Y. Kim, V.J. Fulari, Effect of different electrolytes and deposition time on the supercapacitor properties of nanoflake-like Co(OH)2 electrodes. Ultrason. Sonochem. 51, 49–57 (2019). https://doi.org/10.1016/j.ultsonch.2018.09.003
D. Singh, R.G. Jadhav, A.K. Das, Electrodeposition of binder-free peptide/Co(OH)2 nanohybrid electrodes for solid-state symmetric supercapacitors. Energy Fuels 35, 16152–16161 (2021). https://doi.org/10.1021/acs.energyfuels.1c01850
J.S. Santos, F. Trivinho-Strixino, E.C. Pereira, Investigation of Co(OH)2 formation during cobalt electrodeposition using a chemometric procedure. Surf. Coat. Technol. 205, 2585–2589 (2010). https://doi.org/10.1016/j.surfcoat.2010.10.005
T. Nguyen, M. Boudard, M. Carmezim et al., Layered Ni(OH)2-Co(OH)2 films prepared by electrodeposition as charge storage electrodes for hybrid supercapacitors. Sci. Rep. 7, 39980 (2017). https://doi.org/10.1038/srep39980
S. Tang, X. Li, M. Courté, J. Peng, D. Fichou, Hierarchical Cu(OH)2@Co(OH)2 nanotrees for water oxidation electrolysis. ChemCatChem 20, 4038–4043 (2020). https://doi.org/10.1002/cctc.202000558
V. Gupta, T. Kusahara, H. Toyama, S. Gupta, N. Miura, Potentiostatically deposited nanostructured α-Co(OH)2: a high performance electrode material for redox-capacitors. Electrochem. Commun. 9, 15–23 (2007). https://doi.org/10.1016/j.elecom.2007.06.041
S.D. Dhas, P.S. Maldar, M.D. Patil, S.A. Mane, M.R. Waikar, R.G. Sonkawade, A.V. Moholkar, Fabrication of efficient electrochemical capacitors rooted in sol-gel derived NiMn2O4 nanoparticles. J. Electroanal. Chem. 897, 1572–6657 (2021). https://doi.org/10.1016/j.jelechem.2021.115548
S.D. Dhas, P.S. Maldar, M.D. Patil, M. Waikar, R.G. Sonkawade, S. Chakarvarti, S. Shinde, D. Kim, A. Moholkar, Probing the electrochemical properties of NiMn2O4 nanoparticles as prominent electrode materials for supercapacitor applications. Mater. Sci. Eng. B 271, 115298 (2021). https://doi.org/10.1016/j.mseb.2021.115298
Y. Ma, X. Xie, W. Yang et al., Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors. Adv. Compos. Hybrid Mater. 4, 906–924 (2021). https://doi.org/10.1007/s42114-021-00358-2
D. Wu, X. Xie, Y. Ma, J. Zhang, C. Hou, X. Sun, X. Yang, Y. Zhang, H. Kimura, W. Du, Morphology controlled hierarchical NiS/carbon hexahedrons derived from nitrilotriacetic acid-assembly strategy for high-performance hybrid supercapacitors. Chem. Eng. J. 433, 133673 (2022). https://doi.org/10.1016/j.cej.2021.133673
V. Sunil, A. Yasin, B. Pal, I. Misnon, C. Karuppiah, C.-C. Yang, R. Jose, Tailoring the charge storability of commercial activated carbon through surface treatment. J. Energy Storage 55, 105809 (2022). https://doi.org/10.1016/j.est.2022.105809
Acknowledgements
The author Dr. Suprimkumar D. Dhas is thankful to the Chhatrapati Shahu Maharaj Research Training and Human Development Institute (SARTHI), Pune, Maharashtra, for providing funding. Moreover, all the authors acknowledge the Physics Instrumentation Facility Centre (PIFC), Department of Physics, Shivaji University, Kolhapur, for characterization facilities. The financial assistance provided through DST-PURSE Phase II (2018–2022) and UGC DSA Phase II (2018–2033) is highly acknowledged.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
RTP: Writing—Original draft preparation, Conceptualization, Supervision. ASP: Data curation, Investigation. NBW: Visualization, and Software. SDD: Editing and Resources, Conceptualization, Supervision. TTB: Validation. VJF: Funding acquisition, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
We recognize the importance of maintaining the confidentiality and anonymity of our research participants. All personal information and data collected during the research process are treated with utmost confidentiality. Identifiable information is stored securely and is only accessible to authorized researchers. In our publications, we ensure that individuals cannot be identified directly or indirectly, unless explicit consent has been obtained or where it is essential for the research purposes.
Informed consent
We prioritize obtaining informed consent from all participants involved in our studies. Prior to their participation, we provide detailed information about the purpose of the research, procedures involved, potential risks and benefits, and any other relevant information. Participants are given the opportunity to ask questions and provide voluntary consent before their inclusion in the study. We respect the autonomy and privacy of participants, ensuring their right to withdraw from the study at any time without prejudice.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, R.T., Patil, A.S., Dhas, S.D. et al. Effect of deposition potential and time substitution for Co(OH)2 on controlled synthesis and electrochemical performance for electrochemical supercapacitor. J Mater Sci: Mater Electron 34, 2297 (2023). https://doi.org/10.1007/s10854-023-11436-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11436-0