Abstract
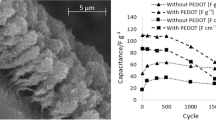
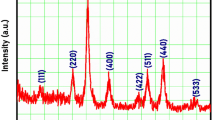
Manganese and Nickel oxides were electrodeposited onto Nickel foam by potentiodynamic (10, 25 and 50 cycles), potentiostatic and galvanostatic modes and the effects of different electrodeposition techniques on the elemental compositions and their supercapactive behaviour were studied to optimise the most appropriate electrodeposition technique for supercapacitor application. The structural properties, morphology and elemental analysis were studied by X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM) accompanied by Energy-Dispersive X-Ray Analysis (EDX). The electrodes’ functional groups were analysed via Fourier Transform-Infrared Spectroscopy (FT-IR). Their electrochemical supercapactive performance were assessed by calculating the areal capacitance from cyclic voltammograms (CV), from Galvanostatic charge-discharge Curves (GCD), and their behaviour was accessed by Electrochemical impedance spectroscopy (EIS) analyses in 0.1 M KOH. The electrochemical results specified among the different electrode MN10, MN25, MN50 (potentiodynamic electrodeposition), MNCA (electrodeposition via chronoamperometry) and MNCP (electrodeposition via chronopotentiometry); MN25 delivered the highest areal capacitance areal capacitance 256.08 F cm−2, with energy density 12.81 Wh cm−2 and power density 150.71 W cm−2 with the capacitance retention percentage of 80.5% at 5 Acm−2 after 5000 cycles.
















Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
M. Hoel, S. Kverndokk, Res. Energy Econ. 18, 115 (1996). https://doi.org/10.1016/0928-7655(96)00005-X
T.N. Veziroğlu, S. Şahi, Energy Convers. Manag. 49, 1820 (2008). https://doi.org/10.1016/j.enconman.2007.08.015
M. Höök, X. Tang, Energy Policy. 52, 797 (2013). https://doi.org/10.1016/j.enpol.2012.10.046
A. Raveendran, M. Chandran, S.M. Wabaidur, M.A. Islam, R. Dhanusuraman, VK Ponnusamy, Fuel. 324, 124424 (2022). https://doi.org/10.1016/j.fuel.2022.124424
M. Chandran, A. Raveendran, A. Thomas, M. Vinoba, S.K. Jeong, M. Bhagiyalakshmi, Synth. Met. 293, 117260 (2023). https://doi.org/10.1016/j.synthmet.2022.117260
J.B. Goodenough, H.D. Abruna, M.V. Buchanan, Basic research needs for electrical energy storage. Report of the basic energy sciences workshop on electrical energy storage, 2–4 April 2007. https://doi.org/10.2172/935429
J.W. Duay, Electrochemical Synthesis, transformation, and characterization of MnO2 nanowire arrays for supercapacitor electrodes (2013). http://hdl.handle.net/1903/14499
R. Dhanusuraman, P. Chahal, A. Raveendran et al., J. Energy Storage. 60, 106554 (2023). https://doi.org/10.1016/j.est.2022.106554
X. Lin, M. Salari, L.M.R. Arava, P.M. Ajayan, M.W. Grinstaff, Chem. Soc. Rev. 45, 5848 (2016). https://doi.org/10.1039/C6CS00012F
PN Pintauro, Polym. Rev. 55, 201 (2015). https://doi.org/10.1080/15583724.2015.1031378
M. Chandran, I. Shamna, A. Anusha, M. Bhagiyalakshmi, SN Appl. Sci. (2019). https://doi.org/10.1007/s42452-019-0509-1
M. Chandran, A. Thomas, A. Raveendran, M. Vinoba, M. Bhagiyalakshmi, J. Energy Storage. 30, 101446 (2020). https://doi.org/10.1016/j.est.2020.101446
Z. Wu, Y. Zhu, X. Ji, C.E. Banks, Nanomater. Adv. Batteries Supercapacitors (2016). https://doi.org/10.1007/978-3-319-26082-2_9
B. De, S. Banerjee, K.D. Verma, T. Pal, P. Manna, K.K. Kar, Handbook of Nanocomposite Supercapacitor Materials II: Performance (Springer, Berlin, 2020). https://doi.org/10.1007/978-3-030-52359-6_4
M. Muthuselvi, K. Jeyasubramanian, G. Hikku et al., Int. J. Energy Res. 45, 8255 (2021). https://doi.org/10.1002/er.6441
W.H. Low, P.S. Khiew, S.S. Lim, C.W. Siong, E.R. Ezeigwe, J. Alloys Compd. 775, 1324 (2019). https://doi.org/10.1016/j.jallcom.2018.10.102
D. Lincot, Thin Solid Films. 487, 40 (2005). https://doi.org/10.1016/j.tsf.2005.01.032
W. Schwarzacher, Electrochem. Soc. Interface. 15, 32 (2006). https://doi.org/10.1149/2.F08061IF
G.A. Ali, M.M. Yusoff, Y.H. Ng, H.N. Lim, K.F. Chong, Curr. Appl. Phys. 15, 1143 (2015). https://doi.org/10.1016/j.cap.2015.06.022
H.Y. Lee, J.B. Goodenough, J. Solid State Chem. 144, 220 (1999). https://doi.org/10.1006/jssc.1998.8128
S. Devaraj, N. Munichandraiah, Electrochem. Solid-State Lett. 8, A373 (2005). https://doi.org/10.1149/1.1922869
D. Dubal, D. Dhawale, T. Gujar, C. Lokhande, Appl. Surf. Sci. 257, 3378 (2011). https://doi.org/10.1016/j.apsusc.2010.11.028
E. Muthusankar, D. Ragupathy, Mater. Lett. 241, 144 (2019). https://doi.org/10.1016/j.matlet.2019.01.071
M. Jayachandran, A. Rose, T. Maiyalagan, N. Poongodi, T. Vijayakumar, Electrochim. Acta. 366, 137412 (2021). https://doi.org/10.1016/j.electacta.2020.137412
L. Hu, W. Chen, X. Xie et al., ACS nano. 5, 8904 (2011). https://doi.org/10.1021/nn203085j
P. Tang, Y. Zhao, C. Xu, Electrochim. Acta. 89, 300 (2013). https://doi.org/10.1016/j.electacta.2012.11.034
M. Arvani, J. Keskinen, D. Lupo, M. Honkanen, J. Energy Storage. 29, 101384 (2020). https://doi.org/10.1016/j.est.2020.101384
T. Gujar, V. Shinde, C. Lokhande, W.-Y. Kim, K.-D. Jung, O.-S. Joo, Electrochem. Commun. 9, 504 (2007). https://doi.org/10.1016/j.elecom.2006.10.017
S. Rahimi, S. Shahrokhian, H. Hosseini, J. Electroanal. Chem. 810, 78 (2018). https://doi.org/10.1016/j.jelechem.2018.01.004
V. Kovalenko, V. Kotok, I. Kovalenko, (2018) Восточно-Европейский журнал передовых технологий, 56
N.A. Salleh, S. Kheawhom, A.A. Mohamad, Arab. J. Chem. 13, 6838 (2020). https://doi.org/10.1016/j.arabjc.2020.06.036
X. Zhang, X. Wang, L. Jiang, H. Wu, C. Wu, J Su, J. Power Sources. 216, 290 (2012). https://doi.org/10.1016/j.jpowsour.2012.05.090
C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang, J. Zhang, Chem. Soc. Rev. 44, 7484 (2015). https://doi.org/10.1039/C5CS00303B
M.J. Carmezim, C.F. Santos, Metal Oxides Supercapacitors. (2017). https://doi.org/10.1016/B978-0-12-810464-4.00003-6
J. Yin, J. Park, Microporous Mesoporous Mater. 200, 61 (2014). https://doi.org/10.1016/j.micromeso.2014.08.016
L. Gu, Y. Wang, R. Lu, L. Guan, X. Peng, J Sha, J. Mater. Chem. A 2, 7161 (2014). https://doi.org/10.1039/C4TA00205A
M. Chandran, A. Raveendran, M. Vinoba, B.K. Vijayan, M. Bhagiyalakshmi, Ceram. Int. 47, 26847 (2021). https://doi.org/10.1016/j.ceramint.2021.06.093
H. Jeong, L.K. Kwac, C.G. Hong, H.G. Kim, Mater. Sci. Eng.: C 118, 111510 (2021). https://doi.org/10.1016/j.msec.2020.111510
M Mylarappa, VV Lakshmi, KV Mahesh, H Nagaswarupa, N Raghavendra (2016) IOP Conference series: materials science and engineering, IOP Publishing, https://doi.org/10.1016/j.matpr.2017.09.152
C.J. Clarke, G.J. Browning, S.W. Donne, Electrochim. Acta. 51, 5773 (2006). https://doi.org/10.1016/j.electacta.2006.03.013
A. Sonavane, A. Inamdar, P. Shinde, H. Deshmukh, R. Patil, P. Patil, J. Alloys Compd. 489, 667 (2010). https://doi.org/10.1016/j.jallcom.2009.09.146
D.T. Dam, X. Wang, J.-M. Lee, Nano Energy. 2, 1303 (2013). https://doi.org/10.1016/j.nanoen.2013.06.011
A. Huang, M.F. El-Kady, X. Chang et al., Adv. Energy Mater. 11, 2100768 (2021). https://doi.org/10.1002/aenm.202100768
K. Allado, M. Liu, A. Jayapalan, D. Arvapalli, K. Nowlin, Energy Fuels 35, 8396 (2021). https://doi.org/10.1021/acs.energyfuels.1c00556
S. Ardizzone, G. Fregonara, S. Trasatti, Electrochim. Acta. 35, 263 (1990). https://doi.org/10.1016/0013-4686(90)85068-X
J. Duay, S.A. Sherrill, Z. Gui, E. Gillette, S.B. Lee, Acs Nano. 7, 1200 (2013). https://doi.org/10.1021/acs.energyfuels.1c00556
J. Wang, J. Polleux, J. Lim, B. Dunn, J. Phys. Chem. C 111, 14925 (2007). https://doi.org/10.1021/jp074464w
K. Brezesinski, J. Wang, J. Haetge et al., J. Am. Chem. Soc. 132, 6982 (2010). https://doi.org/10.1021/ja9106385
R. Gummow, A. De Kock, M. Thackeray, Solid State Ionics. 69, 59 (1994). https://doi.org/10.1016/0167-2738(94)90450-2
S. Sivakkumar, J.M. Ko, D.Y. Kim, B. Kim, Electrochim. Acta 52, 7377 (2007). https://doi.org/10.1016/j.electacta.2007.06.023
S. Singhal, A. Shukla, J. Solid State Electrochem. 24, 1271 (2020). https://doi.org/10.1007/s10008-020-04615-0
A. Zhang, R. Zhao, L Hu, et al., Adv. Energy Mater. 11, 2101412 (2021). https://doi.org/10.1002/aenm.202101412
A. Zhang, R. Gao, L Hu, et al., Chem. Eng. J. 417, 129186 (2021). https://doi.org/10.1016/j.cej.2021.129186
H. Duan, Z. Zhao, J. Lu et al., ACS Appl. Mater. Interfaces. 13, 33083 (2021). https://doi.org/10.1021/acsami.1c08161
X. Tan, S. Liu, Q. Guo et al., Int. J. Energy Res. 44, 4556 (2020). https://doi.org/10.1002/er.5235
H. Zhang, J. Wei, Y Yan, et al., J. Power Sources. 450, 227616 (2020). https://doi.org/10.1016/j.jpowsour.2019.227616
Y. Liu, N. Wang, C. Yang, W Hu, Ceram. Int. 42, 11411 (2016). https://doi.org/10.1016/j.ceramint.2016.04.071
L. Su, L. Gao, Q. Du et al., J. Alloys Compd. 749, 900 (2018). https://doi.org/10.1016/j.jallcom.2018.03.353
Y. Pan, H. Gao, M. Zhang, L. Li, G. Wang, X. Shan, J. Colloid Interface Sci. 497, 50 (2017). https://doi.org/10.1016/j.jcis.2017.02.053
Acknowledgements
Authors are grateful to the Researchers Supporting Project Number (RSP2023R326), King Saud University, Riyadh, Saudi Arabia. Also, the authors would like to thank the basic research support from the National Institute of Technology Puducherry, Karaikal, India.
Funding
This work was supported by King Saud University (Grant No: RSP2023R326).
Author information
Authors and Affiliations
Contributions
AR performed the investigation, MC contributed to data interpretation, MRS and SMW project administration, and funding acquisition, ME contributed to the writing of the original draft and RD contributed to writing, reviewing, and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declaration of interest.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raveendran, A., Chandran, M., Siddiqui, M.R. et al. Different electrodeposition techniques of manganese and nickel oxide on nickel foam and their effect on improved supercapacitor behaviour: a comparative study. J Mater Sci: Mater Electron 34, 2018 (2023). https://doi.org/10.1007/s10854-023-11416-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11416-4