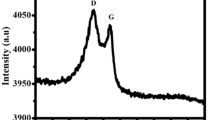
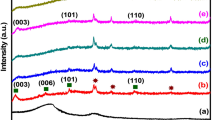
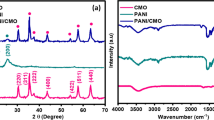
Abstract
Recent applications of supercapacitors in modern society are limited due to their poor electrochemical properties like energy density and cyclic stability, etc. One of the effective approaches to boost the electrochemical performance is to couple the metal oxide with metal and carbon nanostructures. In this study, hybrid ternary nanocomposite, comprised of palladium, activated carbon, and nickel oxide nanoparticles (Pd-AC@NiO), has been synthesized through a simple approach. Here, metal-carbon material plays a significant role in augmenting the specific surface area, while the existence of NiO nanoparticles provides the electroactive sites for energy storage. Consequently, the as-prepared Pd-AC@NiO nanocomposite provides excellent electrochemical performance compared to the as-prepared NiO nanoparticles as an electroactive material. When the hybrid ternary nanocomposite is used as an electroactive material for supercapacitor, it displays an outstanding specific capacitance of 1539 F/g at a current density of 5 A/g in a three-electrode system. Moreover, in terms of energy and power, the as-prepared hybrid ternary nanocomposite electrode demonstrates a high energy density of 34.19 Wh/kg and power density of 1000 W/kg. Additionally, the resulting hybrid ternary nanocomposite electrode shows excellent cycling stability with the capacity retention of 95.5% after 5000 cycles. Thus, these outcomes suggest that the proposed composite material has tremendous electrochemical properties. It deliberated as a promising electrode for the development of energy storage devices with high energy and power densities.







Similar content being viewed by others
References
Gur TM (2018) Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ Sci 11(10):2696–2767
Aneke M, Wang M (2016) Energy storage technologies and real life applications- a state of the art review. Appl Energy 179:350–377
Mahlia TMI, Saktisahdan TJ, Jannifar A, Hasan MH, Matseelar HSC (2014) A review of available methods and development on energy storage: technology update. Renew Sust Energ Rev 33:532–545
Yang Z-R, Wang S-Q, Wang J, Zhou A-J, Xu C-W (2017) Pd supported on carbon containing nickel, nitrogen and sulfur for ethanol electrooxidation. Sci Rep 7(1):15479
Libich J, Maca J, Vondrak J, Cech O, Sedlarikova M (2018) Supercapacitors: properties and applications. Journal of Energy Storage 17:224–227
Muzaffar A, Ahamed MB, Deshmukh K, Thirumalai J (2019) A review on recent advances in hybrid supercapacitors: design, fabrication and applications. Renew Sust Energ Rev 101:123–145
Iro ZS, Subramani C, Dash SS (2016) A brief review on electrode materials for supercapacitors. Int J Electrochem Sci 11:10628–10643
Raza W, Ali F, Raza N, Luo Y, Kim K-H, Yang J, Kumar S, Mehmood A, Kwon EE (2018) Recent advancements in supercapacitors technology. Nano Energy 52:441–473
Miller EE, Hua Y, Tezel FH (2018) Materials for energy storage: review of electrode materials and methods of increasing capacitance for supercapacitors. Journal of Energy Storage 20:30–40
Hao P, Zhao Z, Li L, Tuan C-C, Li H, Sang Y, Jiang H, Wong CP, Liu H (2015) The hybrid nanostructure of MnCo2O4.5nanoneedle/carbon aerogel for symmetric supercapacitors with high energy density. Nanoscale 7(34):14401–14412
Vadiyar MM, Bhise SC, Patil SK, Kolekar SS, Shelke AR, Deshpande NG, Chang J-Y, Ghule KS, Ghule AV (2016) Contact angle measurements: a preliminary diagnostic tool for evaluating the performance of ZnFe2O4nano-flake based supercapacitors. Chem Commun 52(12):2557–2560
Fan Z, Yan J, Wei T, Zhi L, Ning G, Li T, Wei F (2011) Asymmetric supercapacitors based on grapheme/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv Funct Mater 21(12):2366–2375
Shi Q, Cha Y, Song Y, Lee J-I, Zhu C, Li X, Song M-K, Du D, Lin Y (2016) 3D graphene-based hybrid materials: synthesis and applications in energy storage and conversion. Nanoscale 8(34):15414–15447
Zhen L, Fan B, Junjie W, Weiwei Y, Liang W, Zhiwen C, Dengyu P, Minghong W (2018) Boosting the energy storage densities of supercapacitors by incorporating N-doped grapheme quantum dots into cubic porous carbon. Nanoscale 10:22871–22883
Padmanathan N, Selladurai S, Razeeb KM (2015) Ultra-fast rate capability of a symmetric supercapacitor with a hierarchical Co3O4 nanowire/nanoflower hybrid structure in non-aqueous electrolyte. RSC Adv 5(17):12700–12709
Shaikh JS, Pawar RC, Devan RS, Ma YR, Salvi PP, Kolekar SS, Patil PS (2011) Synthesis and characterization of Ru doped CuO thin films for supercapacitor based on bronsted acidic ionic liquid. Electrochim Acta 56(5):2127–2134
Hodaei A, Dezfuli AS, Naderi HR (2018) A high-performance supercapacitor based on N-doped TiO2 nanoparticles. J Mater Sci Mater Electron 29:14596–14604
Xia X, Zhang Y, Chao D, Guan C, Zhang Y, Li L, Ge X, Bacho IM, Tu J, Fan HJ (2014) Solution synthesis of metal oxides for electrochemical energy storage applications. Nanoscale 6(10):5008–5048
Cao F, Pan GX, Xia XH, Tang PS, Chen HF (2014) Synthesis of hierarchical porous NiO nanotube arrays for supercapacitor application. J Power Sources 264:161–167
Yu W, Jiang X, Ding S, Li BQ (2014) Preparation and electrochemical characteristics of porous hollow spheres of NiO nanosheet as electrodes of supercapacitors. J Power Sources 256:440–448
Yuan G, Liu Y, Yue M, Li H, Liu E, Huang Y, Kong D (2014) Cu-doped NiO for aqueous asymmetric electrochemical capacitors. Ceram Int 40(7):9101–9105
Nagamuthu S, Ryu K-S (2019) Synthesis of Ag/NiO honeycomb structured nanoarrays as the electrode material for high-performance asymmetric supercapacitor devices. Sci Rep 9(1):4864
Chen J, Peng X, Song L, Zhang L, Liu X, Luo J (2018) Facile synthesis of Al-doped NiOnanosheet arrays for high-performance supercapacitors. R Soc Open Sci 5(11):180842
Wang Y, Su Q (2016) Synthesis of NiO/Ag nanocomposites by micro-emulsion method and the capacitance performance as electrodes. J Mater Sci Mater Electron 27:4752–4759
Vijayakumar S, Nagamuthu S, Muralidharan G (2013) Porous NiO/C nanocomposites as electrode material for electrochemical supercapacitors. ACS Sustain Chem Eng 9:1110–1118
Chen W, Gui D, Liu J (2016) Nickel oxide/graphene aerogel nanocomposite as a supercapacitor electrode material with extremely wide working potential window. Electrochim Acta 222:1424–1429
Yan Y, Wang T, Li X, Pang H, Xue H (2017) Noble metal-based materials in high-performance supercapacitors. Inorg.Chem.Front. 4(1):33–51
Wu JB, Li ZG, Lin Y (2011) Porous NiO/Ag composite film for electrochemical capacitor application. Electrochim Acta 56(5):2116–2121
Qu B, Hu L, Chen Y, Li C, Li Q, Wang Y, Wei W, Chen L, Wang T (2013) Rational design of Au-NiO hierarchical structures with enhanced rate performance for supercapacitors. J Mater Chem A 1(24):7023–7026
Kalambate PK, Rawool CR, Karna SP, Srivastava AK (2018) Nitrogen-doped grapheme/palladium nanoparticles/porous poluaniline ternary composite as an efficient electrode material for high performance supercapacitor. Materials Science for Energy Technologies 2:246–257
Jiang W, Zhang K, Wei L, Yu D, Wei J, Chen Y (2013) Hybrid ternary rice paper-manganese oxide-carbon nanotube nanocomposites for flexible supercapacitors. Nanoscale 5(22):11108–11117
Mingjia Z, Chengcheng X, Jiangtian L, Ming L, Nianqiang W (2013) Nanostructured carbon-metal oxide composite electrodes for supercapacitors: a review. Nanoscale 5:72–88
Bose S, Kuila T, Mishra AK, Rajasekar R, Kim NH, Lee JH (2012) Carbon-based nanostructured materials and their composites as supercapacitors electrodes. J Mater Chem 22(3):767–784
Chen W, He Y, Li X, Zhou J, Zhang Z, Zhao C, Gong C, Li S, Pan X, Xie E (2013) Facilitated charge transport in ternary interconnected electrodes for flexible supercapacitors with excellent power characteristics. Nanoscale 5(23):11733–11741
Sk MM, Yue CY, Ghosh K, Jena RK (2016) Review on advances in porous nanostructured nickel oxides and their composite electrodes for high-performance supercapacitors. J Power Sources 308:121–140
Winkler K, Grodzka E, D’Souza F, Balch AL (2007) Two-component films of fullerene and palladium as materials for electrochemical capacitors. J Electrochem Soc 154(4):K1–K10
Dar RA, Giri L, Karna SP, Srivastava AK (2016) Performance of palladium nanoparticle-graphene composite as an efficient electrode material for electrochemical double layer capacitors. Electrochim Acta 196:547–557
Jin Y, Zhao J, Li F, Jia W, Liang D, Chen H, Li R, Hu J, Ni J, Wu T, Zhong D (2016) Nitrogen-doped graphene supported palladium-nickel nanoparticles with enhanced catalytic performance for formic acid oxidation. Electrochim Acta 220:83–90
Dhanushkotti R, Pulidindi IN, Arumugam P, Chinnathambi M (2018) Pd-NiO decorated multiwalled carbon nanotubes supported on reduced graphene oxide as an efficient electrocatalyst for ethanol oxidation in alkaline medium. Appl Surf Sci 442:787–796
Wang H-Q, Fan X-P, Zhang X-H, Huang Y-G, Wu Q, Pan Q-C, Li Q-Y (2017) In situ growth of NiO nanoparticles on carbon paper as a cathode for rechargeable Li-O2 batteries. RSC Adv 7(38):23328–23333
Acknowledgments
S.S. acknowledges the fellowship support from the University Grant Commission (UGC) India. The authors are thankful to the CRF and NRF facilities provided by IIT Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3008 kb)
Rights and permissions
About this article
Cite this article
Singhal, S., Shukla, A. Improved electrochemical performance of supercapacitors by utilizing ternary Pd-AC-doped NiO nanostructure as an electrode material. J Solid State Electrochem 24, 1271–1282 (2020). https://doi.org/10.1007/s10008-020-04615-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04615-0