Abstract
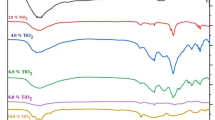
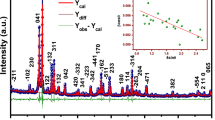
This manuscript focuses on investigating how the ratio of glass modifier to former (m/f) affects the properties of silver borotellurite glass. Upon preparing the glasses with the formula Ag2SO4-TeO2-0.6B2O3, spectroscopic and dielectric analysis was performed on the samples. Crystalline phase is present in samples with an m/f ratio of more than 0.8, while amorphous phase is proven by the absence of prominent peaks in XRD spectra for samples with a m/f ratio of less than 0.8. The densities of the samples became greater with the addition of the various modifiers to the mixture. The mixing caused values for the optical band gap to drop while increasing values for the Urbach energy. The FTIR spectra made the discovery that the structure of the glass had a number of borate and tellurite groups. On the basis of conductivity measurements, an investigation of the change in AC, DC, and activation energy with modifier to former ratio is carried out. The results of the dielectric investigations suggested that an increase in the m/f ratio of Ag + could potentially lead to an improvement in the ionic conductivity of the material.



















Similar content being viewed by others
Data availability
The datasets supporting the conclusions of this article are included within the article.
References
H.M.H. Zakaly, S.A.M. Issa, H.O. Tekin, A. Badawi, H.A. Saudi, A.M.A. Henaish, Y.S. Rammah, An experimental evaluation of CdO/PbO-B2O3 glasses containing neodymium oxide: Structure, electrical conductivity, and gamma-ray resistance. Mater. Res. Bull. 151, 111828 (2022). https://doi.org/10.1016/j.materresbull.2022.111828
P. Giridhar, H.J. Seo, S. Sailaja, M. Bhushan Reddy, C. Nageshwer Raju, B. Sudhaker Reddy, Spectroscopic investigations of Eu3+ and Tb3+: Cadmium lead boro-tellurite glasses. Glass Phys. Chem. 38, 77–84 (2012). https://doi.org/10.1134/S1087659612010075
A.K. Yadav, P. Singh, Impedance spectroscopic studies of mixed alkali tellurite glasses. J. Mater. Sci. 26, 9443–9450 (2015). https://doi.org/10.1007/s10854-015-3375-7
Y.B. Saddeek, K.A. Aly, K.S. Shaaban, A.M. Ali, M.A. Sayed, Elastic, Optical and structural features of wide range of CdO- Na2B4O7 glasses. Mater. Res. Express 5(6), 065204 (2018). https://doi.org/10.1088/2053-1591/aac93f
G. Sangeetha, K. ChandraSekhar, A. Hameed, G. Ramadevudu, M. Narasimha Chary, M. Shareefuddin, Influence of CaO on the structure of zinc sodium tetra borate glassescontaining Cu2+ ions. J. Non-Cryst. Solids 563, 120784 (2021). https://doi.org/10.1016/j.jnoncrysol.2021.120784
Y.B. Saddeek, M.H. Zakaly, K. Chandra Sekhar, A.M. Issa, T. Alharbi, A. Badawi, M. Shareefuddin, Investigations of mechanical and radiation shielding properties of BaTiO3-modifed cadmium alkali borate glass. Appl. Phys. A 128, 260 (2022). https://doi.org/10.1007/s00339-022-05413-3
K. Chandra Sekhar, N. Narsimlu, M.S. Al-Buriahi, H.A. Yakout, I.O. Olarinoye, S. Alomairy, M. Shareefuddin, Synthesis, optical, and radiation attenuation properties of CaF2-TeO2-Na2B4O7-CuO glass system for advanced shielding applications. Eur. Phys. J. Plus 136, 903 (2021). https://doi.org/10.1140/epjp/s13360-021-01906-x
P. Nageswar Rao, E. Ramesh Kumar, C.S.R. Madhireddy, A. Prabhakar Reddy, K. Krishnamurthy Goud, B. Appa Rao, Optical studies of AgI–Ag2SO4–TeO2–B2O3 glass system. Mater. Today 5(2018), 26329–26338 (2018). https://doi.org/10.1016/j.matpr.2018.08.084
K. Chandra Sekhar, M. Raheem, N. Narsimlu, U. Deshpande, V.G. Sathe, M. Shareefuddin, The effect of the addition of CaF2 and PbF2 on boro-tellurite glasses doped with chromium ions. Mater. Res. Express 6, 125206 (2019). https://doi.org/10.1088/2053-1591/ab619f
A.A. El-Moneim, R. El-Mallawany, Y.B. Saddeek, Nb2O5–TeO2 and Nb2O5–Li2O–TeO2 glasses: Evaluation of elastic properties. J. Non-Cryst. Solids 575, 121229 (2022). https://doi.org/10.1016/j.jnoncrysol.2021.121229
F.A. Abdel-Wahab, A.M. Fayad, M. Abdel-Baki, Role of non-bridging oxygen defect in the ionic conductivity and associated oxygen trap centers in lead-borate oxide glass: Effect of structural substitution of PbO for Ag2O and Li2O modifiers. J. Non-Cryst. Solids 500, 84–91 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.06.033
S. Bhattacharya, A. Ghosh, Relaxation of silver ions in superionic borate glasses. Chem. Phys. Lett. 424(4–6), 295–299 (2006). https://doi.org/10.1016/j.cplett.2006.04.077
P. Naresha, N. Narsimlu, C. Srinivas, M. Shareefuddin, K. Siva Kumar, Ag2O doped bioactive glasses: An investigation on the antibacterial, optical, structural and impedance studies. J. Non Cryst. Solids 549, 120361 (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120361
T.V.N. Keerti Kut, S. Marijan, J. Pisk, A. Venkata Sekhar, A. Siva, S. Reddy, N. Venkatramaiah, G.N. Raju, Impact of silver ions on dielectric properties and conductivity of lithium silicate glass system mixed with red lead. J. Non Cryst. Solids 588, 121641 (2022). https://doi.org/10.1016/j.jnoncrysol.2022.121641
S. Rada, M. Rada, R.V. Erhan, V. Bodnarchuk, L. Barbu Tudoran, E. Culea, Hetero geneities in the silver oxide-lead-germanate glasses. J. Alloys Compd. 770, 395–404 (2019). https://doi.org/10.1016/j.jallcom.2018.08.128
V. Prasad, L. Pavić, A. Moguš-Milanković, A.S.S. Reddy, Y. Gandhi, V.R. Kumar, G.N. Raju, N. Veeraiah, Influence of silver ion concentration on dielectric characteristics of Li2O-Nb2O5-P2O5 glasses. J. Alloys Compd 773, 654–665 (2019). https://doi.org/10.1016/j.jallcom.2018.09.161
J. Ashok, M. Kostrzewa, A. Ingram, N. Venkatramaiah, M.S. Reddy, V.R. Kumar, M. Piasecki, N. Veeraiah, Structural and dielectric features of silver doped sodium antimonate glass ceramics. J. Alloys Compd 791, 278–295 (2019). https://doi.org/10.1016/j.jallcom.2019.03.228
M. Nagarjuna, P.R. Rao, Y. Gandhi, V.R. Kumar, N. Veeraiah, Electrical conduction and other related properties of silver ion doped LiF–V2O5–P2O5 glass system. Phys. B 405, 668–677 (2010)
P. Naresh, N. Narsimlu, C. Srinivas, M.D. Shareefuddin, K. Siva Kumar, Ag2O doped bioactive glasses: An investigation on the antibacterial, optical, structural and impedance studies. J. Non Cryst. Solids 549, 120361 (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120361
S. Bhattacharya, A. Ghosh, Relaxation of silver ions in superionic borate glasses. Chem. Phys. Letters 424, 295 (2006). https://doi.org/10.1016/j.cplett.2006.04.077
C. Calahoo, L. Wondraczek, Ionic glasses: Structure, properties and classification. J. Non Cryst. Solids X 8, 100054 (2020). https://doi.org/10.1016/j.nocx.2020.100054
G.V.J. Gouda, B. Eraiah, R.V. Anavekar, Ionic conductivity of praseodymium doped silver-borate glasses. J. Alloys Compnds. 620, 192–196 (2015). https://doi.org/10.1016/j.jallcom.2014.09.019
Y.B. Saddeek, M.S. Gaafar, Physical and structural properties of some bismuth borate glasses. Mat. Chem. Phys. 115, 280–286 (2009). https://doi.org/10.1016/j.matchemphys.2008.12.004
K.C. Sekhar, A. Hameed, N. Narsimlu, J.S. Alzahrani, M.A. Alothman, I.O. Olarinoye, M.S. Al-Buriahi, M.D. Shareefuddin, Synthesis, optical, structural, and radiation transmission properties of PbO/Bi2O3/B2O3/Fe2O3 glasses: An experimental and in silico study. Opt. Mater. 117, 111173 (2021). https://doi.org/10.1016/j.optmat.2021.111173
S.G. Ramadevudu, M.N. Chary, M. Shareefuddin, Physical and spectroscopic studies of Cr3+ doped mixed alkaline earth oxide borate glasses. Mater. Phys. Chem. 186, 382–389 (2017). https://doi.org/10.1016/j.matchemphys.2016.11.009
Y. Saddeek, L. Abd El Latif, Effect of TeO2 on the elastic moduli of sodium borate glasses. Phys. B 348, 475 (2004). https://doi.org/10.1016/j.physb.2004.02.001
K. El-Egili, A. Oraby, The structure and electrical properties of lithium borate glasses containing thallic oxide. J. Phys. Condens. Matter 8, 8959 (1996). https://doi.org/10.1088/0953-8984/8/46/003
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92, 1324 (1953). https://doi.org/10.1103/PhysRev.92.1324
A. Davis, N.F. Mott, Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 22, 903–922 (1970). https://doi.org/10.1080/14786437008221061
J. Tauc, A. Menth, States in the gap. J. Non-Cryst. Solids 8, 569–585 (1972). https://doi.org/10.1016/0022-3093(72)90194-9
K.C. Sekhar, A. Hameed, M.N. Chary, M. Shareefuddin, Physical, optical and electron paramagnetic resonance studies of PbBr 2-PbO-B2O3 glasses containing Cu2+ ions. IOP Conf. Ser. 149, 012167 (2016). https://doi.org/10.1088/1757-899X/149/1/012167
S. Thakur, V. Thakur, A. Kaur, L. Singh, Synthesis and the study of structural, thermal and optical properties of (100–x)Bi2O3-x(BaO-TiO2) glass system. Optik 223, 165646 (2020). https://doi.org/10.1016/j.ijleo.2020.165646
K. Chandra Sekhar, M. Shareefuddin, A. El-Denglawey, Y.B. Saddeek, Structural and optical properties of BaTiO3 modified cadmium alkali borate glasses. Phys. Scr. 97, 035704 (2022). https://doi.org/10.1088/1402-4896/ac53c7
V. Dimitrov, S. Sakka, Electronic oxide polarizability and optical basicity of simple oxides. I. J. Appl. Phys. 79, 736 (1996). https://doi.org/10.1063/1.360962
V. Dimitrov, T. Komatshu, Classification of simple oxides: A Palarizability approach. J. Solid State Chem. 163, 100 (2002). https://doi.org/10.1006/jssc.2001.9378
J.A. Duffy, Chemical bonding in the oxides of the elements: A new appraisal. J. Solid State Chem. 62, 145 (1986). https://doi.org/10.1016/0022-4596(86)90225-2
P. Nageswar Rao, E. Ramesh Kumar, B. Appa Rao, Structural and transport studies of CdI2-dopedsilver borotellurite fastion-conducting system. J. Solid State Electrochem. 22, 3863–3871 (2018). https://doi.org/10.1007/s10008-018-4094-9
L. Haritha, K. Chandra Sekhar, R. Nagaraju, G. Ramadevudu, V.G. Sathe, M.D. Shareefuddin, Effect of metal fluorides on chromium ions doped bismuth borate glasses for optical applications. Chin. Phys. B (2019). https://doi.org/10.1088/1674-1056/28/3/038101
B. Ashok, K.C. Sekhar, B.S. Chary, G. Ramadevudu, M.N. Chary, M.D. Shareefuddin, Physical and structural study of Al2O3–NaBr–B2O3–CuO glasses. Indian J Phys (2021). https://doi.org/10.1007/s12648-021-02048-7
S.A. Suthanthiraraj, R. Sarumathi, Electrical and structural study of new antimony iodide doped silver sulphate electrolyte. Ionics 19(8), 1145–1153 (2013). https://doi.org/10.1007/s11581-012-0826-5
M.Z. Iqbal, R. Rafiuddin, Electrical conductivity, dielectric, modulus and optical studies of Ag2SO4 and TiO2 composite solid electrolytes. Mater. Sci. Forum 842, 76–87 (2016). https://doi.org/10.4028/www.scientific.net/MSF.842.76
E. Lefterova, P. Angelov, V. Ilcheva, T. Petkova1, Y. Dimitriev “Investigation of Agi-Ag2so4-Teo2 glasses and glass ceramics” Nanoscience& Nanotechnology, 4eds. E. Balabanova, I. Dragieva, Heron Press, Sofia, 2004
S. Rada, M. Culea, E. Culea, Structure of TeO2 B2O3 glasses inferred from infrared spectroscopy and DFT calculations. J. Non-Cryst Solids 354, 5491–5495 (2008). https://doi.org/10.1016/j.jnoncrysol.2008.09.009
V. Kozhukharov, S. Nikolav, M. Marinov, T. Troev, Studies of glass structure in the TeO2- Fe2O3 system. Mater. Res. Bull. 14, 735 (1979). https://doi.org/10.1016/0025-5408(79)90132-6
S. Dariush, K. Shomalian, Band gap determination by Absorption Spectrum Fitting method (ASF) and structural properties of different compositions of (60–x) V2O5–40TeO2–xSb2O3 glasses. J. Non-Cryst. Solids. 355, 1597–1601 (2009). https://doi.org/10.1016/j.jnoncrysol.2009.06.003
S. Rada, E. Culea, M. Rada, P. Pescuta, V. Maties, Structural and electronic properties of tellurite glasses. J Mater Sci 44, 3235–3240 (2009). https://doi.org/10.1007/s10853-009-3433-8
G.D. Chryssikos, E.I. Kamitsos, A.P. Patsis, Synthesis and characterization of new solid electrolyte conductors of lithium ions. Solid State Ionics 1, 177–186 (1980). https://doi.org/10.1016/0167-2738(80)90002-8
G.D. Chrissicos, E.I. Kamitsos, A.P. Patsis, Effect of Li2SO4 on the structure of Li2O-B2O3 glasses. J. Non-Cryst. Solids 202, 222 (1996). https://doi.org/10.1016/0022-3093(96)00200-1
A. Prabhakar Reddy, P.N. Rao, M.C.S. Reddy, B. Appa Rao, N. Veeraiah, Second harmonic generation and spectroscopic characteristics of TiO2doped Li2O–Al2O3–B2O3 glass matrix. Appl. Phys. A 126, 689 (2020). https://doi.org/10.1007/s00339-020-03879-7
A. Bhargava, R.L. Snyder, R.A. Condrate, The Raman and infrared spectra of the glasses in the system BaO-TiO2-B2O3. Mater. Res. Bull. 22, 1603 (1987). https://doi.org/10.1016/0025-5408(87)90002-X
A.K. Jonscher, The ‘universal’ dielectric response. Nature 267, 673 (1977). https://doi.org/10.1038/267673a0
A.K. Jonscher, The universal dielectric response: a review of data their new interpretation (Chelsea Dielectrics Group, London, 1978)
N.K. Karan, B. Natesan, R.S. Katiyar, Structural and lithium ion transport studies in borophosphate glasses. Solid State Ionics 177, 1429–1436 (2006). https://doi.org/10.1016/j.ssi.2006.07.032
A. Ghosh, A. Pan, Scaling of the Conductivity Spectra in Ionic Glasses: Dependence on the Structure. Phys. Rev. Let. 84(10), 2188–2190 (2000). https://doi.org/10.1103/PhysRevLett.84.2188
P. Nageswar Rao, E. Ramesh Kumar, B. Appa Rao, Effect of quenching rate on electrical conductivity and glass formation of AgI–Ag2SO4–TeO2–B2O3 system. J. Mater. Sci. 29, 11247–11257 (2018). https://doi.org/10.1007/s10854-018-9211-0
P.N. Rao, E. Ramesh Kumar, B. Appa Rao, Structural, electrical, and transport number studies of AgI-doped silver borotellurite fast ion conducting glass system. Ionics 24(12), 3885–3895 (2018). https://doi.org/10.1007/s11581-018-2550-2
A.K. Joncher, Dielectric relaxation in solids (Chesla dielectric press, London, 1983)
M. Mohamad, A.K. Yamada, T. Okuda, Ionic conduction and relaxation in KSn2F5 fluoride ion conductor. Physica B 339(2–3), 94–100 (2003). https://doi.org/10.1016/j.physb2003.08.056
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
PNR: Material preparation, data collection, conceptualization, investigation, validation. KCS: Material preparation, data collection, conceptualization, investigation, validation. TR: Investigation, methodology, writing—original draft, MCSR: Investigation, methodology, writing—original draft. KVR: Investigation, methodology, writing—original draft. MS: Investigation, writing—review and editing, supervision. BAR: Investigation, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors whose names are listed immediately below the title of the manuscript certify that they have NO affiliations with or involvement in any organization or entity with any financial interest(such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rao, P.N., Sekhar, K.C., Ramesh, T. et al. Effect of glass modifier to former ratio on spectroscopic and transport properties of silver boro-tellurite glass system. J Mater Sci: Mater Electron 34, 1756 (2023). https://doi.org/10.1007/s10854-023-11165-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11165-4