Abstract
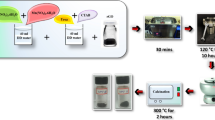
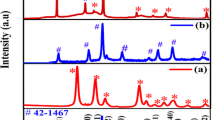
Metal oxides exhibit high theoretical specific capacitance due to its multiple oxidation states and when combined with reduced graphene oxide (rGO) may produce synergistic effects. Hence various metal oxides with rGO have been studied for supercapacitor properties. In this work, a two-step hydrothermal approach was followed to produce Co3O4 – Mn2O3 / rGO nanocomposite with the average crystallite size of 14 nm. Functional groups in the rGO allow for the nucleation to occur on its surface which results in the successful metal oxide nanocomposite formation. The formed metal oxides has average particle diameter of 29 nm with surface area of 14.139 m2/g. Mesoporous nature of the nanocomposite was obtained with an average pore diameter below 20 nm. The presence of more active sites for the electrolyte ions in the electrode surface results in higher specific capacitance of 663 F/g at 1 A/g and low electrode resistance of 0.39 Ω. Thus the electrochemical studies reveal the diffusion controlled intercalation process over a potential range of 0–0.6 V.









Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
S. Huang, X. Zhu, S. Sarkar, Y. Zhao, Challenges and opportunities of supercapacitors. APL Mater. (2019). https://doi.org/10.1063/1.5116146. 7 100901.
P. Dhruba, K. Chatterjee, Arun, Nandi, A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 9, 15880–15918 (2021). https://doi.org/10.1039/D1TA02505H
S. Banerjee, B. De, P. Sinha, J. Cherusseri, K.K. Kar, Applications of Supercapacitors, in Handbook of Nanocomposite Supercapacitor Materials I. ed. by K. Kar (Springer Series in Materials Science, 300 Springer, Cham, 2020), pp.54–87
D. Wu, X. Xie, Y. Zhang, D. Zhang, W. Du, X. Zhang, B. Wang, MnO2/Carbon Composites for Supercapacitor: synthesis and Electrochemical Performance. Front. Mater. 7, 2 (2020). https://doi.org/10.3389/fmats.2020.00002
M. Yaseen, M.A.K. Khattak, M. Humayun, M. Usman, S.S. Shah, S. Bibi, B.S.U. Hasnain, S.M. Ahmad, A. Khan, N. Shah, A.A. Tahir, H. Ullah, A review of supercapacitors: materials design, modification, and applications. Energies 14, 7779 (2021). https://doi.org/10.3390/en14227779
F. Xueyang Wang, D. Yuan, J. Xue, J. Liu, Y. Wei, J. Wang, Q. Wang, Q. Qu, Zhang, MnO2 Nanoflowers decorated on ZIF-8-ZnO with Ni foam supportfor high-performance supercapacitors (Nano Mat, Chem, 2022). https://doi.org/10.1002/cnma.202200243
M. Aqib Muzaffar, Basheer Ahamed, J. Kalim Deshmukh Thirumalai, A review on recent advances in hybrid supercapacitors: design, fabrication and applications. Renew. Sustainable Energy Rev. 101, 123–145 (2019). https://doi.org/10.1016/j.rser.2018.10.026
Y. Pan, K. Xu, C. Wu, Recent progress in supercapacitors based on the advanced carbon electrodes. nanotechnol Rev. 8, 299–314 (2019). https://doi.org/10.1515/ntrev-2019-0029
M.B. Askari, P. Salarizadeh, A. Beheshti-Marnani, A. Di, NiO–Co3O4–rGO as an efficient electrode material for supercapacitors and direct alcoholic fuel cells. Adv. Mater. Interfaces 8, 2100149 (2021). https://doi.org/10.1002/admi.202100149
H.J. Trinity Rabecca, A.J. Clement Lourduraj, Synthesis of reduced graphene oxide and a study on its electrochemical performance for supercapacitor applications. Mater. Today: Proc. 68P3, 335–340 (2022). https://doi.org/10.1016/j.matpr.2022.05.541
J.P.B. Hajar Ghannam, Silva, C. Adil, Effect of ZnO surface morphology on its electrochemical performance. RSC Adv. 11, 23346–23354 (2021). https://doi.org/10.1039/D1RA03653J
W. Zhang, H. Xu, F. Xie, X. Ma, N. Bo, M. Chen, H. Zhang, Y. Zhang, D. Long, General synthesis of ultrafine metal oxide/reduced graphene oxide nanocomposites for ultrahigh-flux nanofiltration membrane. Nat. Commun. 13, 471 (2022). https://doi.org/10.1038/s41467-022-28180-4
A. Kostuch, J. Grybos, S. Wierzbicki, Z. Sojka, K. Kruczała, Selectivity of mixed Iron-cobalt spinels deposited on a N,S-Doped Mesoporous Carbon support in the Oxygen reduction reaction in Alkaline Media. Materials. 14, 820 (2021). https://doi.org/10.3390/ma14040820
S. Wu Yinbo, C. Ruirui, Facile synthesis of cobalt oxide as an efficient electrocatalyst for hydrogen evolution reaction. Front. Chem 8, 386 (2020). https://doi.org/10.3389/fchem.2020.00386
E. Arciga-Duran, Y. Meas, J.J. Pérez-Bueno, J.C. Ballesteros, G. Trejo, Electrochemical synthesis of Co3O4-x films for their application as oxygen evolution reaction electrocatalysts: role of oxygen vacancies. J. Electrochem. Soc. 165, H3178 (2018). https://doi.org/10.1149/2.0261804jes
T. Shen, Z. Zhao, Q. Zhong, Y. Qin, Z.-X. Guo, Preparation of graphene/Au aerogel film through the hydrothermal process and application for H 2 O 2 detection. RSC Adv. 9, 13042–13047 (2019). https://doi.org/10.1039/C9RA00516A
S. Xiao, P. Xu, Q. Peng, J. Chen, J. Huang, Wang, Faming, N. Noor, Layer-by-Layer Assembly of Polyelectrolyte Multilayer onto PET Fabric for highly tunable dyeing with Water Soluble Dyestuffs. Polymer. 9, 735 (2017). https://doi.org/10.3390/polym9120735
P. Scherrer, Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges Wiss Göttingen. 26, 98–100 (1918). https://doi.org/http://eudml.org/doc/59018
J.I. Langford, A.J.C. Wilson, Scherrer after Sixty Years: a Survey and some New results in the determination of Crystallite size. J. Appl. Cryst. 11, 102–113 (1978). https://doi.org/10.1107/s0021889878012844
V. Uvarov, I. Popov, Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charac. 85, 111–123 (2013). https://doi.org/10.1016/j.matchar.2013.09.002
C. Sengottaiyan, R. Jayavel, R.G. Shrestha, P.H. Jonathan, A. Katsuhiko, K.S. Lok, Electrochemical Supercapacitance Properties of reduced graphene Oxide/Mn2O3:Co3O4 nanocomposite. J. Inorg. Organomet. Polym. 27, 576–585 (2017). https://doi.org/10.1007/s10904-017-0501-4
H. Cao, X. Peng, M. Zhao, P. Liu, B. Xu, J. Guo, Oxygen functional groups improve the energy storage performance graphene electrochemical supercapacitors. RSC Adv. 8, 2858–2865 (2018). https://doi.org/10.1039/C7RA12425B
P. Balu, I.V. Asharani, D. Thirumalai, Catalytic degradation of hazardous textile dyes by iron oxide nanoparticles prepared from Raphanus sativus leaves’ extract: a greener approach. J. Mater. Sci: Mater. Electron. 31, 10669–10676 (2020). https://doi.org/10.1007/s10854-020-03616-z
J.-C. Shu, X.-Y. Huang, Mao-Sheng Cao, Assembling 3D flower-like Co3O4-MWCNT architecture for optimizing low-frequency microwave absorption. Carbon. 174, 638–646 (2021). https://doi.org/10.1016/j.carbon.2020.11.087
M.S. Yadav, Synthesis and characterization of Mn2O3 – Mn3O4 nanoparticles and activated charcoal based nanocomposite for supercapacitor electrode application. J. Energy Storage. (2020). https://doi.org/10.1016/j.est.2019.101079
H. Rahaman, R. Laha, S. Ghosh, D. Maiti, Fabrication of Mn2O3 nanorods: an efficient catalyst for selective transformation of alcohol to aldehyde. RSC Adv. 5, 33923–33929 (2015). https://doi.org/10.1039/c5ra02504d
C.P. Lee, B.T. Murti, P.K. Yang, F. Rossi, C.R. Carraro, Maboudian, Cobalt Oxide-Decorated Silicon Carbide Nano-Tree array electrode for Micro-Supercapacitor Application. Materials. 14, 4514 (2021). https://doi.org/10.3390/ma14164514
Ahmed M. Abdel-Raoof, Ayman OE. Osman, Ebrahim A. El-Desouky, Ashraf Abdel-Fattah, Rady F. Abdul-Kareem, Elsayed Elgazzar, Fabrication of an (α-Mn 2 O 3:Co)-decorated CNT highly sensitive screen printed electrode for the optimization and electrochemical determination of cyclobenzaprine hydrochloride using response surface methodology. RSC Adv. 10, 24985–24993 (2020). https://doi.org/10.1039/D0RA05106C
Gokuladeepan Periyasamy, Indrajit M. Patil, Bhalchandra Kakade, J. Pandiyarasan Veluswamy, Hiroya Ikeda Archana, Karthigeyan Annamalai, Reduced graphene oxide-wrapped α-Mn2O3/α-MnO2 nanowires for electrocatalytic oxygen reduction in alkaline medium. Mater. Sci: Mater. Electron. 33, 8644–8654 (2022). https://doi.org/10.1007/s10854-021-06721-9
Y. Dong, N. Xiaoyu, W. Song, D. Wang, Chen, Liqiang, Yuan, Fulong, Y. Zhu, Facile synthesis of Vanadium oxide/reduced graphene oxide composite catalysts for enhanced hydroxylation of benzene to phenol. Catalysts 6, 74 (2016). https://doi.org/10.3390/catal6050074
K.J. Noémie Elgrishi, B.D. Rountree, E.S. McCarthy, T.T. Rountree, Eisenhart, J.L. Dempsey, A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 2:197–206 (2018). https://doi.org/10.1021/acs.jchemed.7b00361
S.S. Pradeepa, P. Rajkumar, K. Diwakar, K. Sutharthani, R. Subadevi, M. Sivakumar, A facile one-pot hydrothermal synthesis of Zn, Mn co-doped NiCo2O4 as an efficient electrode for supercapacitor applications. Chemistryselect 6(27), 6851–6862 (2021). https://doi.org/10.1002/slct.202101708
M. Isacfranklin, B. Jansi Rani, P. Senthil Kumar, R. Yuvakkumar, G. Ravi, A. Manigandan, Mariyappan Thambidurai, Cuong Dang, Dhayalan Velauthapillai, Electrochemical energy storage and conversion applications of CoSn(OH)6 materials. Int. J. Hydrogen Energy 47, 41948–41955 (2022). https://doi.org/10.1016/j.ijhydene.2021.08.001
Y. Yang, Z.-. Pan, Y.-Y. Wang, Ma, Yuan, C. Li, Y.-. Lu, X.-L. Wu, Ionic-liquid-bifunctional wrapping of Ultrafine SnO2 nanocrystals into N-doped Graphene Networks: high pseudocapacitive Sodium Storage and High-Performance Sodium-Ion full cell. Nanoscale. 11, 14616–14624 (2019). https://doi.org/10.1039/C9NR02542A
H. Fangyan Liu, L. Su, H. Jin, X. Zhang, W. Chu, Yang, Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 505, 796–804 (2017). https://doi.org/10.1016/j.jcis.2017.06.058
C.I. Priyadharsini, G. Marimuthu, T. Pazhanivel, P.M. Anbarasan, V. Aroulmoji, V. Siva, L. Mohana, Sol–Gel synthesis of Co3O4 nanoparticles as an electrode material for supercapacitor applications. J. Sol-Gel Sci. Technol. 96, 416–422 (2020). https://doi.org/10.1007/s10971-020-05393-x
V. Venkatachalam, A. Alsalme, A. Alswieleh, R. Jayavel, Shape controlled synthesis of rod-like Co3O4 nanostructures as high-performance electrodes for supercapacitor applications. J. Mater. Sci. : Mater. Electron. 29, 6059–6067 (2018). https://doi.org/10.1007/s10854-018-8580-8
J. Xu, Y. Sun, M. Lu, L. Wang, J. Zhang, J. Qian, E.J. Kim, Fabrication of porous Mn2O3 microsheet arrays on nickel foam as high–rate electrodes for supercapacitors. J. Alloys Compd. 717, 108–115 (2017). https://doi.org/10.1016/j.jallcom.2017.04.239
S.A. Ansari, N. Parveen, H. Mahfoz Kotb, Adil Alshoaibi, Hydrothermally derived three-dimensional porous hollow double-walled Mn2O3 nanocubes as superior electrode materials for supercapacitor applications. Electrochim. Acta. 355, 136783 (2020). https://doi.org/10.1016/j.electacta.2020.136783
G.P. Ojha, B. Pant, A. Muthurasu, S.-H. Chae, S.-J. Park, T. Kim, H.-Y. Kim, Three-dimensionally assembled manganese oxide ultrathin nanowires: prospective electrode material for asymmetric supercapacitors. Energy. (2019). https://doi.org/10.1016/j.energy.2019.116066. ,188:116066
S. Ouksaphea Pech, Electrochemical performances of electrospun carbon nanofibers, interconnected carbon nanofibers, and carbon-manganese oxide composite nanofibers. J. Alloys Compd. 781, 541–552 (2019). https://doi.org/10.1016/j.jallcom.2018.12.088
Y. Jiang, C. He, S. Qiu, J. Zhang, X. Wang, Yingkui Yang, Scalable mechanochemical coupling of homogeneous Co3O4 nanocrystals onto in-situ exfoliated graphene sheets for asymmetric supercapacitors. Chem. Eng. J. 397, 125503 (2020). https://doi.org/10.1016/j.cej.2020.125503
S. Ramesh, K. Karuppasamy, H.S. Kim, H.S. Kim, J.H. Kim, Hierarchical flowerlike 3D nanostructure of Co3O4@MnO2/N-doped graphene oxide (NGO) hybrid composite for a high-performance supercapacitor. Sci. Rep. 8, 16543 (2018). https://doi.org/10.1038/s41598-018-34905-7
N. Imen Abidli, X.R. Souissi, Novoa, Corrosion inhibition of steel by prickly pear: extraction, characterisation and electrochemical studies. Rev. Roum Chim 65, 353–360 (2020). https://doi.org/10.33224/rrch.2020.65.4.04
Acknowledgements
This work was supported by SJCRG (2022–2023), St. Josehph’s College (autonomous), Tiruchirappalli-620002, India.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
TRHJ – Conceptualization, Methodology, Investigation, Writing – original draft. PY – Investigation, Writing – review and editing. MS- Resources, Writing – review and editing. Clement LAJ – Writing – review and editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Not applicable.
Ethical approval
This article does not contain any studies involving human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trinity Rabecca, H.J., Priyajanani, Y., Manivannan, S. et al. Investigation of electrochemical behavior of Co3O4–Mn2O3/rGO nanocomposite for supercapacitor applications. J Mater Sci: Mater Electron 34, 1390 (2023). https://doi.org/10.1007/s10854-023-10810-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10810-2