Abstract
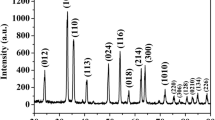
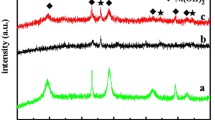
Mesoporous α-Fe2O3 nanowires have been prepared through solid-state thermal conversion of ferrous oxalate dihydrate precursor for supercapacitor application. The possible growth mechanism of the FeC2O4·2H2O nanowires was proposed based on a series of time-dependent experiments. The specific surface area and pore size distribution of the mesoporous α-Fe2O3 nanowires were calculated to be about 70.6 m2 g−1 and 2.5 nm, respectively. Furthermore, electrochemical measurements demonstrate that the as-prepared mesoporous α-Fe2O3 nanowire electrode delivers a high specific capacitance up to 267.5 F g−1 at 2 A g−1 and good cycle performance (87% capacitance retention under 2000 cycles). The excellent supercapacitor performance of the α-Fe2O3 nanowires can be ascribed mainly to the unique mesoporous structure with large specific surface area, which provide fast electron/ion transfer path as well as large reaction surface area.









Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
M.F. EL-Kady, V. Strong, S. Dubin, R.B. Kaner, Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 335(6074), 1326–1330 (2012). https://doi.org/10.1126/science.1216744
Z. Yu, L. Tetard, L. Zhai, J. Thomas, Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 8(3), 702–730 (2015). https://doi.org/10.1039/C4EE03229B
X. Ren, M.G. Li, L.Y. Qiu, X. Guo, F.Y. Tian, G.H. Han, W.W. Yang, Y.S. Yu, Cationic vacancies and interface engineering on crystalline–amorphous gamma-phase Ni–Co oxyhydroxides achieve ultrahigh mass/areal/volumetric energy density flexible all-solid-state asymmetric supercapacitor. J. Mater. Chem. A 11, 5754–5765 (2023). https://doi.org/10.1039/d2ta09035j
X. Guo, M.G. Li, L.Y. Qiu, F.Y. Tian, L. He, S. Geng, Y.Q. Liu, Y. Song, W.W. Yang, Y.S. Yu, Engineering electron redistribution of bimetallic phosphates with CeO2 enables high-performance overall water splitting. Chem. Eng. J. 453, 139796 (2023). https://doi.org/10.1016/j.cej.2022.139796
M.Y. Gao, F.Y. Tian, Z. Guo, X. Zhang, Z.J. Li, J. Zhou, X. Zhou, Y.S. Yu, W.W. Yang, Mutual-modification effect in adjacent pt nanoparticles and single atoms with sub-nanometer inter-site distances to boost photocatalytic hydrogen evolution. Chem. Eng. J. 446, 137127 (2022). https://doi.org/10.1016/j.cej.2022.137127
X. Zhang, M.Y. Gao, L.Y. Qiu, J. Sheng, W.W. Yang, Y.S. Yu, Sulfur vacancies-induced ‘‘Electron Bridge” in Ni4Mo/Sv–ZnxCd1−xS regulates electron transfer for efficient H2-releasing photocatalysis. J. Energy Chem. 79, 64–71 (2023). https://doi.org/10.1016/j.jechem.2023.01.001
X. Zhang, C.X. Zhu, L.Y. Qiu, M.Y. Gao, F.Y. Tian, Y.Q. Liu, W.W. Yang, Y.S. Yu, Concentrating photoelectrons on sulfur sites of ZnxCd1–xS to active H–OH bond of absorbed water boosts photocatalytic hydrogen generation. Surf. Interfaces 34, 102312 (2022). https://doi.org/10.1016/j.surfin.2022.102312
G.R. Li, H. Xu, X.F. Lu, J.X. Feng, Y.X. Tong, C.Y. Su, Electrochemical synthesis of nanostructured materials for electrochemical energy conversion and storage. Nanoscale 5(10), 4056–4069 (2013). https://doi.org/10.1039/C3NR00607G
J. Xu, Q. Wang, X. Wang, Q. Xiang, B. Hang, D. Chen, G. Shen, Flexible asymmetric supercapacitors based upon Co9S8 nanorod Co3O4@RuO2 nanosheet arrays on carbon cloth. ACS Nano 7(6), 5453–5462 (2013). https://doi.org/10.1021/nn401450s
H. Wang, H. Yi, X. Chen, X. Wang, One-step strategy to three-dimensional graphene/VO2 nanobelt composite hydrogels for high performance supercapacitors. J. Mater. Chem. A 2(4), 1165–1173 (2014). https://doi.org/10.1039/C3TA13932H
Y. Li, J. Xu, T. Feng, Q.F. Yao, J.P. Xie, H. Xia, Fe2O3 nanoneedles on ultrafine nickel nanotube arrays as efficient anode for high-performance asymmetric supercapacitors. Adv. Funct. Mater. 27(14), 1606728 (2017). https://doi.org/10.1002/adfm.201606728
L. Liu, J. Lang, P. Zhang, B. Hu, X. Yan, Facile synthesis of Fe2O3 nano-dots@nitrogen-doped graphene for supercapacitor electrode with ultralong cycle life in KOH electrolyte. ACS Appl. Mater. Interfaces 8(14), 9335–9344 (2016). https://doi.org/10.1021/acsami.6b00225
Z. Wang, C.J. Liu, Preparation and application of iron oxide/graphene based composites for electrochemical energy storage and energy conversion devices: current status and perspective. Nano Energy 11, 277–293 (2015). https://doi.org/10.1016/j.nanoen.2014.10.022
D. Sarkar, G.G. Khan, A.K. Singh, K. Mandal, High-performance pseudocapacitor electrodes based on α-Fe2O3/MnO2 core-shell nanowire heterostructure arrays. J. Phys. Chem. C 117(30), 15523–15531 (2013). https://doi.org/10.1021/jp4039573
P. Zhao, W. Li, G. Wang, B. Yu, X. Li, J. Bai, Z. Ren, Facile hydrothermal fabrication of nitrogen-doped graphene/Fe2O3 composites as high performance electrode materials for supercapacitor. J. Alloys Compd. 604, 87–93 (2014). https://doi.org/10.1021/j.jallcom.2014.03.106
Z. Ma, X. Huang, S. Dou, J. Wu, S. Wang, One-pot synthesis of Fe2O3 nanoparticles on nitrogen-doped graphene as advanced supercapacitor electrode materials. J. Phys. Chem. C 118(31), 17231–17239 (2014). https://doi.org/10.1021/jp502226j
L. Xu, J. Xia, H. Xu, S. Yin, K. Wang, L. Huang, L. Wang, H. Li, Reactable ionic liquid assisted solvothermal synthesis of graphite-like C3N4 hybridized α-Fe2O3 hollow microspheres with enhanced supercapacitive performance. J. Power Sources 245, 866–874 (2014). https://doi.org/10.1016/j.jpowsour.2013.07.014
Q.X. Low, G.W. Ho, Facile structural tuning and compositing of iron oxide-graphene anode towards enhanced supacapacitive performance. Nano Energy 5, 28–35 (2014). https://doi.org/10.1016/j.nanoen.2014.01.002
D. Dollimore, D.L. Griffiths, D. Nicholson, The thermal decomposition of oxalates. Part II. Thermogravimetric analysis of various oxalates in air and in nitrogen. J. Chem. Soc. 488, 2617–2623 (1963). https://doi.org/10.1016/j.nanoen.2014.01.002
E.D. Macklen, Influence of atmosphere on the thermal decomposition of ferrous oxalate dehydrate. J. Inorg. Nucl. Chem. 29, 1229–1234 (1967). https://doi.org/10.1016/0022-1902(67)80362-2
Y.M. Zhao, Y.H. Li, R.Z. Ma, M.J. Roe, D.G. McCartney, Y.Q. Zhu, Growth and characterization of iron oxide nanorods/nanobelts prepared by a simple iron-water reaction. Small 2(3), 422–427 (2006). https://doi.org/10.1002/smll.200500347
H.L. Du, J.Z. Wang, B. Wang, D.Q. Cang, Preparation of cobalt oxalate powders with the presence of a pulsed electromagnetic field. Powder Technol. 199(2), 149–153 (2010). https://doi.org/10.1016/j.powtec.2009.12.015
N. Du, Y.F. Xu, H. Zhang, C.X. Zhai, D.R. Yang, Selective synthesis of Fe2O3 and Fe3O4 nanowires via a single precursor: a general method for metal oxide nanowires. Nanoscale Res. Lett. 5(8), 1295–1300 (2010). https://doi.org/10.1007/s11671-010-9641-y
C.E. Houseroft, Inorganic Chemistry (Pearson Education; Edinburgh Gate, London, 2005)
X.L. Li, J.F. Liu, Y.D. Li, Low-temperature conversion synthesis of M(OH)2 (M=Ni, Co, Fe) nanoflakes and nanorods. Mater. Chem. Phys. 80(1), 222–227 (2003). https://doi.org/10.1016/S0254-0584(02)00488-1
Y.D. Li, H.W. Liao, Y. Ding, Y. Fan, Y. Zhang, Y.T. Qian, Solvothermal elemental direct reaction to CdE (E=S, Se, Te) semiconductor nanorod. Inorg. Chem. 38(7), 1382–1387 (1999). https://doi.org/10.1021/ic980878f
P.H. Zhao, W.L. Li, G. Wang, B.Z. Yu, X.J. Li, J.T. Bai, Z.Y. Ren, Facile hydrothermal fabrication of nitrogen-doped graphene/Fe2O3 composites as high performance electrode materials for supercapacitor. J. Alloy Compd. 604, 87–93 (2014). https://doi.org/10.1016/j.jallcom.2014.03.106
J. Zhao, Z.J. Li, X.C. Yuan, Z. Yang, M. Zhang, A. Meng, Q.D. Li, A high-energy density asymmetric supercapacitor based on Fe2O3 nanoneedle arrays and NiCo2O4/Ni(OH)2 hybrid nanosheet arrays grown on SiC nanowire networks as free-standing advanced electrodes. Adv. Energy Mater. 8, 1702787 (2018). https://doi.org/10.1002/aenm.201702787
Q. Tang, W. Wang, G. Wang, The perfect matching between the low-cost Fe2O3 nanowire anode and the NiO nanoflake cathode significantly enhances the energy density of asymmetric supercapacitors. J. Mater. Chem. A 3(12), 6662–6670 (2015). https://doi.org/10.1039/CSTA00328H
X.J. Yang, H.M. Sun, L.S. Zhang, L.J. Zhao, J.S. Lian, Q. Jiang, High efficient photo-fenton catalyst of α-Fe2O3/MoS2 hierarchical nanoheterostructures: reutilization for supercapacitors. Sci. Rep. 6, 31591 (2016). https://doi.org/10.1038/srep31591
J.C. Huang, S.N. Yang, Y. Xu, X.B. Zhou, X. Jiang, N.N. Shi, D.X. Cao, J.L. Yin, G.L. Wang, Fe2O3 sheets grown on nickel foam as electrode material for electrochemical capacitors. J. Electroanal. Chem. 713, 98–102 (2014). https://doi.org/10.1016/j.jelechem.2013.12.009
Y.D. Dong, L. Xing, F. Hu, A. Umar, X. Wu, α-Fe2O3/rGO nanospindles as electrode materials for supercapacitors with long cycle life. Mater. Res. Bull. 107, 391–396 (2018). https://doi.org/10.1016/j.materresbull.2018.07.038
S. Yang, X. Song, P. Zhang, J. Sun, L. Gao, Self-assembled α-Fe2O3 mesocrystals-graphene nanohybrid for enhanced electrochemical capacitors. Small 10(11), 2270–2279 (2014). https://doi.org/10.1002/smll.201303922
K. Deori, S.K. Ujjain, R.K. Sharma, S. Deka, Morphology controlled synthesis of nanoporous Co3O4 nanostructures and their charge storage characteristics in supercapacitors. ACS Appl. Mater. Interfaces 5(21), 10665–10672 (2013). https://doi.org/10.1021/am4027482
Y. Wang, M.M. Zhang, D.H. Pan, Y. Li, T.J. Ma, J.M. Xie, Nitrogen/sulfur co-doped grapheme networks uniformly coupled N-Fe2O3 nanoparticles achieving enhanced supercapacitor. Electrochim. Acta 266, 242–253 (2018). https://doi.org/10.1016/j.electacta.2018.02.040
C.L. Long, T. Wei, J. Yan, L.L. Jiang, Z.J. Fan, Supercapacitors based on graphene-supported iron nanosheets as negative electrode materials. ACS Nano 7(12), 11325–11332 (2013). https://doi.org/10.1021/nn405192s
U.M. Patil, K.V. Gurav, V.J. Fulari, C.D. Lokhande, O.S. Joo, Characterization of honeycomb-like “β-Ni(OH)2” thin films synthesized by chemical bath deposition method and their supercapacitor application. J. Power Sources 188(1), 338–342 (2009). https://doi.org/10.1016/j.jpowsour.2008.11.136
N.K. Chaudhari, Cube-like α-Fe2O3 supported on ordered multimodal porous carbon as high performance electrode material for supercapacitors. ChemSusChem 7(11), 3102–3111 (2014). https://doi.org/10.1002/cssc.201402526
B.J. Lokhande, R.C. Ambare, R.S. Mane, S.R. Bharadwaj, Concentration-dependent electrochemical supercapacitive performance of Fe2O3. Curr. Appl. Phys. 13(6), 985–989 (2013). https://doi.org/10.1016/j.cap.2013.01.047
K.Y. Xie, J. Li, Y.Q. Lai, W. Lu, Z. Zhang, Y.X. Liu, L.M. Zhou, H.T. Huang, Highly ordered iron oxide nanotube arrays as electrodes for electrochemical energy storage. Electrochem. Commun. 13(6), 657–660 (2011). https://doi.org/10.1016/j.elecom.2011.03.040
K.K. Lee, S. Deng, H.M. Fan, S. Mhaisalkar, H.R. Tan, E.S. Tok, K.P. Loh, W.S. Chin, Sow, α-Fe2O3 nanotubes-reduced graphene oxide composites as synergistic electrochemical capacitor materials. Nanoscale 4(9), 2958–2961 (2012). https://doi.org/10.1039/C2NR11902A
S. Sun, J. Lang, R. Wang, L. Kong, X. Li, X. Yan, Identifying pseudocapacitance of Fe2O3 in an ionic liquid and its application in asymmetric supercapacitors. J. Mater. Chem. A 2(35), 14550–14556 (2014). https://doi.org/10.1039/C4TA02026J
P.M. Padwal, S.L. Kadam, S.M. Mane, S.B. Kulkarni, Enhanced specific capacitance and supercapacitive properties of polyaniline–iron oxide (PANI–Fe2O3) composite electrode material. J. Mater. Sci. 51(23), 10499–10505 (2016). https://doi.org/10.1007/s10853-016-0270-4
X. Zheng, X.Q. Yan, Y.H. Sun, Y.S. Yu, G.J. Zhang, Y.W. Shen, Q.J. Liang, Q.L. Liao, Y. Zhang, Temperature-dependent electrochemical capacitive performance of the α-Fe2O3 hollow nanoshuttles as supercapacitor electrodes. J. Colloid Interface Sci. 466, 291–296 (2016). https://doi.org/10.1016/j.jcis.2015.12.024
S. Lee, H. Kim, H.M. Jung, Interfacial generation of plates assembled with α-Fe2O3 nano-flakes for electrochemical capacitors. J. Electroanal. Chem. 770, 44–49 (2016). https://doi.org/10.1016/j.jelechem.2016.03.035
L.C. Yue, S.G. Zhang, H.Q. Zhao, M. Wang, D.F. Wang, J. Mi, Microwave-assisted one-pot synthesis of Fe2O3/CNTs composite as supercapacitor electrode materials. J. Alloy Compd. 765, 1263–1266 (2018). https://doi.org/10.1016/j.jallcom.2018.06.283
M.Y. Zhu, J.R. Kan, J.M. Pan, W.J. Tong, Q. Chen, J.C. Wang, One-pot hydrothermal fabrication of α-Fe2O3@C nanocomposites for electrochemical energy storage. J. Energy Chem. 28, 1–8 (2019). https://doi.org/10.1016/j.jechem.2017.09.021
S. Shivakumara, T.R. Penki, N. Munichandraiah, Preparation and electrochemical performance of porous hematite (α-Fe2O3) nanostructures as supercapacitor electrode material. J. Solid State Electrochem. 18(4), 1057–1066 (2014). https://doi.org/10.1007/s10008-013-2355-1
S. Shivakumara, T.R. Penki, N. Munichandraiah, Synthesis and characterization of porous flowerlike α-Fe2O3 nanostructures for supercapacitor application. ECS Electrochem. Lett. 2(7), A60–A62 (2013). https://doi.org/10.1149/2.002307eel
B.P. Prasanna, D.N. Avadhani, M.S. Raghu, K.K. Yogesh, Synthesis of polyaniline/α-Fe2O3 nanocomposite electrode material for supercapacitor applications. Mater. Today Commun. 12, 782–787 (2017). https://doi.org/10.1016/j.mtcomm.2017.07.002
D. Wang, Q. Wang, T. Wang, Controlled synthesis of mesoporous hematite nanostructures and their application as electrochemical capacitor electrodes. Nanotechnology 22(13), 135604 (2011). https://doi.org/10.1088/0957-4484/22/13/135604
T.Z. Shi, Y.L. Feng, T. Peng, B.G. Yuan, Sea urchin-shaped Fe2O3 coupled with 2D MXene nanosheets as negative electrode for high-performance asymmetric supercapacitors. Electrochim. Acta 381, 138245 (2021). https://doi.org/10.1016/j.electacta.2021.138245
S.B. Tian, B.L. Zhang, D. Han, Z.Q. Gong, X.Y. Li, Fe2O3/porous carbon composite derived from oily sludge waste as an advanced anode material for supercapacitor application. Nanomaterials 12, 3819 (2022). https://doi.org/10.3390/nano12213819
Z.Y. Yu, X.Y. Zhang, L. Wei, X. Guo, MOF-derived porous hollow α-Fe2O3 microboxes modified by silver nanoclusters for enhanced pseudocapacitive storage. Appl. Surf. Sci. 463, 616–625 (2019). https://doi.org/10.1016/j.apsusc.2018.08.262
H.Y. Quan, B.C. Cheng, Y.H. Xiao, S.J. Lei, One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composites for supercapacitor application. Chem. Eng. J. 286, 165–173 (2016). https://doi.org/10.1016/j.CEJ.2015.10.068
S.W. Zahng, B.S. Yin, Z.B. Wang, F. Peter, Super long-life all solid-state asymmetric supercapacitor based on NiO nanosheets and α-Fe2O3 nanorods. Chem. Eng. J. 306, 193–203 (2016). https://doi.org/10.1016/j.cej.2016.07.057
Funding
This work was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant Nos. HZ2021013, KJQN202001341, KJQN202001304, and KJZD-K202001305) and the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0670 and cstc2020jcyj-msxmX0103).
Author information
Authors and Affiliations
Contributions
HW participated in the investigation, data curation, and writing of the original draft, YL participated in the conceptualization, writing, reviewing, & editing of the manuscript, and supervision. WX participated in the investigation. LT participated in the methodology. JS participated in the validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, H., Li, Y., Xiao, W. et al. Preparation and electrochemical properties of mesoporous α-Fe2O3 nanowires for supercapacitor application. J Mater Sci: Mater Electron 34, 1098 (2023). https://doi.org/10.1007/s10854-023-10456-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10456-0