Abstract
This study reports a facile impregnation method for synthesizing Ni-doped TiO2 nanomaterials using P25-TiO2 as a starting material. The as prepared nanomaterials were subjected to structural and optical characterizations and subsequently employed in photovoltaic studies. X-ray diffraction (XRD) and Raman studies confirmed that Ni doping did not alter the anatase and rutile contents of P25-TiO2. Also, the presence of the constituent dopants and their ionic states were confirmed by Energy-Dispersive X-ray (EDX) and X-ray photoelectron (XPS) spectroscopies. Topographic Atomic Force Microscopic (AFM) images illustrated that Ni doping had increased the surface roughness of the TiO2. Optical characterization by UV-Visible spectroscopy revealed that the Ni doping had caused red shift in light absorption due to reduced TiO2 bandgap and improved the dye adsorption on TiO2 films. Then, the photocurrent–photovoltage property of the fabricated devices was investigated and the optimized 0.10 wt% Ni-doped TiO2 photoanode based device exhibited pronounced power conversion efficiency (PCE) of 6.29% under air mass (AM) 1.5 conditions (100 mWcm−2, 1 sun). Improved charge transport properties were also observed by the electrochemical impedance spectroscopic (EIS) study for the device with optimized Ni-doped TiO2 compared to the control device.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dye-sensitized solar cells (DSSCs) have become a significant topic in the field of solar photovoltaic technology for three decades because of their cost-effectiveness, eco-friendliness and working ability under low intensity light conditions [1]. It consists of an electrode with a mesoporous wide bandgap semiconductor sensitized with a light absorbing dye, a redox electrolyte and a counter electrode made up of platinized conductive glass [2]. TiO2 is widely used as the semiconductor in DSSC due to its’ porous nature, non-toxic and uncomplicated preparation methods and ability to easily combine with other metal oxides or dopants [3]. However, its overall performance in the photovoltaic application is still limited due to poor electron mobility of nano porous TiO2 [4] and limited solar spectral response of the dye in the visible region [5, 6]. In the literature, various attempts have been reported to overcome these limitations by placing a compact metal oxide blocking layer on the Transparent Conducting Oxide (TCO), coating the TiO2 film with a thin layer of a wide band gap semiconductor, forming composites with TiO2 electrode, modifying the morphology of nanostructured semiconductors using self-assembled monolayer [7] / insulator [8] and doping/co-doping the TiO2 with other elements [9].
Many studies have reported the promising effect of dopants on the TiO2 based DSSCs. Among the effective dopants, transition metals (Zn [5, 10], Ag [11], Nb [12], W [13], Cu [14], etc.) are found to be superior to non-transition elements as they improve light absorption and electrical conductivity by incorporating new impurity energy levels within the TiO2 band gap or modifying the conduction band or valence band of the TiO2 [15, 16]. Among the transition elements, it is noted that high electrical conductivity of Nickel (Ni) prevents loss of electrons during electron transfer and thus increases the current density and efficiency of DSSCs when used as a dopant [17]. Also, Ni doping on TiO2 improves visible light harvest through a red shift in the wavelength of absorption of the solar radiation [18].
Many studies on the influence of Ni-doped TiO2 on the performance of DSSCs are reported in the literature. Power conversion efficiencies (PCEs) of 2.86%, 3.60% and 4.04% were demonstrated for DSSCs fabricated with Ni-doped TiO2 photoanode, where the doped nanomaterial was synthesized by sol-gel method [19,20,21]. In a separate study, Malik and his group has reported that hydrothermally synthesized Ni-doped TiO2 based device increases the photovoltaic current and hence improves the device efficiency up to 6.72% [22]. A common feature in all the above doping methods is the treatment of Ni dopant with a titanium precursor. Alternatively, doping could be achieved by treating the Ni dopant with pre-made TiO2. Moreover, the method of doping also plays a crucial role in altering the properties of the TiO2 which in turn strongly influences the corresponding DSSC performance.
So far, the treatment of Ni dopant with the pre-made TiO2 (P25-TiO2) nanomaterial and its influence on the device performance have not been investigated. Hence, an attempt was made to synthesize the Ni-doped TiO2 nanomaterial by a facile wet impregnation method using commercially available P25-TiO2 and NiCl2.6H2O. This is a very simple approach which involves introduction of the dopant atoms directly into the pre-made TiO2 lattice in a liquid solvent and subsequent removal of the solvent at high temperature.
2 Materials and methods
2.1 Synthesis of Ni-doped TiO2 nanomaterials
Initially, 1 g of P25-TiO2 nanopowder (21 nm primary particle size, ≥ 99.5% trace metals basis, Sigma-Aldrich) was dispersed into ethanol (> 99%, Sigma Aldrich) and the resulting TiO2 slurry was stirred for 1 h. Then, 0.05, 0.10, 0.50 and 1.00 wt% of Ni2+ solutions were prepared by dissolving the respective amounts of NiCl2.6H2O (99.9%, Sigma-Aldrich) in ethanol separately. Subsequently, the prepared Ni2+ solutions were individually mixed with the TiO2 slurry and stirred vigorously for 2 h to obtain the respective Ni–TiO2 mixtures, which were dried at 100 °C and ground well using Agate mortar and pestle to obtain a fine powder. The above products were calcinated at 500 °C for 2 h and Ni-doped TiO2 nanomaterials were synthesized (Fig. 1). Finally, the DSSC was fabricated as reported in our previous study [23].
2.2 Characterization of the synthesized nanomaterials
The crystalline structures of all the Ni-doped and un-doped TiO2 nanomaterials were explored by X-ray diffraction spectroscopy (XRD, PANalytical-AERIS, Almelo, Netherlands). The diffraction pattern was collected with Cu Kα radiation (λ = 1.5408 Å) at ambient temperature, under the accelerated voltage of 40 kV; emission current of 44 mA; scan range (2θ) between 10° and 90° with a step size of 0.0027° and a scan speed of 4°/min. Raman spectroscopic measurements were analyzed using a laser confocal Raman microscope (Renishaw, UK, Model: Invia). The elemental analysis of the nanomaterials was studied by Energy Dispersive analysis of X-ray technique (Bruker EDX analyser). The surface electronic state of the Ni-doped TiO2 was determined by X-ray photoelectron spectroscopy (XPS, Kratos Axis Ultra DLD). The surface nature of the nanomaterials was investigated using atomic force microscopy (Park XE7, Korea). Absorption spectra of all the synthesized nanomaterials were recorded using a JENWAY 6800 UV-Visible Spectrophotometer (OSA, UK) controlled by Flight Deck software. The photovoltaic performance of all the fabricated devices with an effective area of 0.25 cm2 was studied using Keithley-2400 source meter under simulated irradiation of 1 sun illumination intensity (100 mW/cm2) by 150 W Xe lamp with AM 1.5 filter (Peccell-PEC-L12, Kanagawa, Japan). The charge transport properties of the devices were measured by electrochemical impedance spectroscopy (EIS) at the frequency range from 10−2 to 106 Hz under light condition using a Metrohm Autolab Potentiostat/galvanostat (PGSTAT 128 N, Utrecht, Netherlands) with a FRA 32 M Frequency Response Analyzer (FRA).
3 Results and discussion
3.1 Structural properties of Ni-doped TiO2 nanomaterial
Figure 2a reveals the X-ray diffraction results of the un-doped TiO2 and 0.05, 0.10, 0.50 and 1.00 wt% Ni-doped TiO2 films. The XRD patterns of all the fabricated films exhibited peaks at 25.20°, 37.60°, 48.20°, 53.70°, 55.00°, 62.50°, 68.50°, 70.20°, 74.89° and 82.53° corresponding to the reflection planes of (101), (004), (200), (105), (211), (204), (116), (220), (215) and (224) for anatase TiO2 phase, while peaks at 27.39°, 36.07° and 41.2° correspond to the reflection planes of (110), (101) and (111) for rutile TiO2 phase. The obtained values match well with the anatase XRD JCPDS Card No. 21-1272 and rutile JCPDS Card No. 21-1276) respectively [24].
All Ni-doped and un-doped TiO2 show the same anatase and rutile peaks, confirming that phase transition in TiO2 had not taken place during Ni doping. This could be attributed to the similarity in the ionic radii of Ni2+ (0.072 nm) and Ti4+ (0.068 nm) [25] that leads to replacement of Ti4+ ions in the lattice by Ni2+ ions. The average crystallite size of the synthesized nanomaterials was calculated by the Scherrer equation using anatase (101) plane as reported in our previous study [23]. The approximate crystallite sizes (d) of un-doped, 0.05, 0.10, 0.50 and 1.00 wt% Ni-doped TiO2 nanomaterials were found to be 18.3, 18.2, 17.6, 17.5 and 16.5 nm respectively. The reduction in crystallite size would have resulted due to the shorter Ni–O bond distance (1.87 Å) than that of Ti–O bond (1.94 Å) [26]. Similar inhibitory trends have been reported by Alijani and her co-worker with respect to the growth of crystallite size and anatase-rutile phase transformation due to Ni doping [27]. The absence of crystallite peaks of Ni in the XRD of the synthesized Ni-doped TiO2 may be due to the low concentrations of Ni dopant. Raman spectroscopy was carried out in the range from 100 to 800 cm−1 to further confirm the crystallinity of the synthesized nanomaterials and the results are shown in Fig. 2b. Generally, the Raman spectrum of TiO2 nanomaterial shows five bands corresponding to the six Raman active modes of TiO2. Well resolved Raman peaks with the D4h space group at about 170, 216, and 657 cm−1 (Eg), 539 cm−1 (A1g + B1g), and 420 cm−1 (B1g) corresponding to the anatase phase of TiO2 were observed [23]. The absence of peaks for Ni and crystalline oxides of Ni could be attributed to the low concentration of Ni which is in consistent with the XRD results. A reduction in the intensity of the peaks of Raman spectra was observed with increasing amounts of Ni dopant which indicates the impact of interaction between TiO2 and Ni on the Raman resonance of TiO2. Similar effect of Ni doping on the intensity of TiO2 peaks is reported in the literature [21]. To confirm the existence of Ni dopant in the Ni-doped TiO2 nanomaterials, EDX and XPS spectroscopies were carried out.
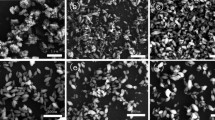
Figure 3a–e exhibit EDX spectra of the un-doped and all Ni-doped TiO2 nanomaterials in the binding energy range from 0.0 to 20.0 KeV. The results reveal the presence of Ti, O and Ni in the Ni-doped TiO2 nanomaterials and the amount of Ni increases with the increasing amount of Ni dopant (Table 1). Further, EDX mapping of the optimized Ni-doped TiO2 (0.10 wt%) illustrates extensive dispersion of Ti, O and Ni elements confirming that Ni is well-distributed on the surface of TiO2 (Fig. 3f). Further, XPS study was performed to confirm the electronic state of the dopant in the Ni-doped TiO2 nanomaterials.
Figure 4 represents XPS spectrum of the optimized 0.10 wt% of Ni-TiO2 nanomaterial consisting of Ti, O, and Ni elements. As reported in our previous study, the 2p3/2 and 2p1/2 peaks in the Ti 2p spectrum are characteristic of titanium dioxide. The peaks located at 455.7 and 461.4 eV belong to Ti 2p spin-orbit splitting photoelectrons of Ti4+ ions. Also, the binding energy at 526.9 eV of O 1s photoelectron confirms the presence of O2− state in the nanoparticles [28]. The peak for the Ni 2p3/2 located at 852.73 eV is characteristic of metallic Ni which could be mainly attributed to the alloy substrate [29]. Moreover, it is reported in the literature that peaks at 858.4 and 870.5 eV represent the Ni 2p3/2 and Ni 2p1/2 photoelectrons respectively which are responsible for the Ni2+ or Ni–O species [30, 31]. Hence, doping of Ni in the TiO2 lattice is confirmed as per the XPS study. Further, it is noteworthy to mention that the crystallite size reduction observed in the XRD study of Ni-doped TiO2 due to Ni–O bond formation is also confirmed by the above XPS results.
Then, the effect of the presence of Ni on the nature of TiO2 surface was examined by atomic force microscopy. Figure 5 shows the 3D and 2D AFM images of the un-doped, 0.05, 0.10, 0.50 and 1.00 wt% of Ni-doped TiO2 nanomaterials coated films.
According to the AFM images, particles are of spherical shape in all samples and the doping did not change the morphology of TiO2. Also, the AFM images confirm that Ni doping reduces crystallite size of the TiO2 and the particle size which further decreases with the increase in dopant concentration, the reason for such observations has already been explained. This is supportive evidence to the XRD results. As a result of the reduction in crystallite size and particle size, the surface roughness (Root mean square) of the film gradually increases from 0.40 μm (for un-doped TiO2) to 0.76 μm (for 1.00 wt% Ni-doped TiO2) (Table 2). Higher surface roughness helps to adsorb more dye molecules due to the increased contact area between TiO2 and the dye molecules layer which in turn facilitates charge transportation in the device. However, it was found that Ni doping beyond 0.10 wt% negatively affects the dye adsorption due to agglomeration of the nanoparticles. In a separate study, Ganesh and his co-researchers has also reported that particles are started to agglomerate in higher Ni content in Ni-doped TiO2 (beyond 0.5 wt%) [32].
3.2 Optical properties of Ni-doped TiO2 nanomaterial
UV-Visible spectroscopic study was performed to measure the light absorption capacities of un-doped TiO2 and 0.05, 0.10, 0.50 and 1.00 wt% Ni-doped TiO2 and estimate their bandgaps (Fig. 6a, b). Ni-doped TiO2 showed a gradual shift to longer wavelengths (red shift) in the UV–Visible absorption spectrum with increase in the dopant concentration compared to the un-doped TiO2 (Fig. 6a). Further, the approximate bandgap values of the above nanomaterials were determined using Tauc plot as reported elsewhere [33, 34], and found to be 3.14, 3.12, 3.08, 2.98 and 2.93 eV for un-doped TiO2, and 0.05, 0.10, 0.50 and 1.00 wt% for Ni-doped TiO2 nanomaterials respectively (Fig. 6b).
The observed bandgap narrowing on Ni doping would have predominantly resulted due to creation of an energy level by 3d orbital of Ni just above the valence band thereby uplifting the valence band of TiO2 which had led to the red shift in the UV-Visible spectrum. It is noteworthy to mention that the influence of Ni (3d) orbital on the conduction band of TiO2 is lower compared to its valence band [35,36,37].
The influence of Ni doping on dye adsorption was analyzed by dipping the un-doped and 0.05, 0.10, 0.50 and 1.00 wt% Ni-doped TiO2 films in the N719 dye for about 12 h and measuring the light absorption ability of the resultant dye coated films by UV-Visible spectroscopy. The Fig. 7 reveals improved light absorption for dye coated Ni-doped TiO2 films and the maximum light absorption was attained on 0.10 wt% Ni doping. The better light absorption of dye coated Ni-doped TiO2 films is ascribed to the increased surface area for dye adsorption because of Ni doping. This was confirmed by increase in surface roughness and slight reduction in particle size due to Ni doping as observed in the respective AFM images. However, further increase in the dopant concentration beyond 0.10 wt% Ni reduced light absorption due to agglomeration of the particles which led to reduction in dye adsorption. These dye adsorption capabilities by Ni-doping also affect the PV properties of the DSSCs, probably the JSC value.
3.3 Photovoltaic performance
The photovoltaic performance of the un-doped and all Ni-doped TiO2 photoanodes based DSSCs were analyzed by J–V measurements under air mass (AM) 1.5 conditions (100 mWcm−2, 1 sun) and the results are summarized in Fig. 8a and Table 3. The J–V studies revealed that the PCE, especially the JSC value, of TiO2 based DSSCs is strongly affected by the amount of Ni doping (Fig. 8b). When the wt% of Ni dopant was increased, initially the JSC value showed an increment from 11.90 to 12.40 mA/cm2 for 0.05 wt% Ni dopant compared to the control device and then, it further increased and attained an optimum value of 13.62 mA/cm2 for 0.10 wt% Ni; subsequently, the JSC value showed a descending trend with further increase in Ni wt% (Fig. 8b).
The variation in JSC values could be attributed to the modified surface properties of Ni-doped TiO2 as explained earlier. In this study, the DSSC fabricated with 0.10 wt% Ni-doped TiO2 electrode exhibited the best η of 6.29% which was around 20% improvement compared to the control device (un-doped TiO2 based DSSC, η = 5.02%) under air mass (AM) 1.5 conditions (100 mWcm−2, 1 sun). In addition to the light harvesting ability, charge transport resistance also plays a major role in the device performance.
3.4 Electrochemical impedance spectroscopic (EIS) analysis
The electrochemical impedance spectroscopy was carried out to analyze the interfacial charge transfer properties of 0.10 and 1.00 wt% Ni-doped and un-doped TiO2 based devices. The electron transport properties at each interfacial were evaluated using Nyquist plots of EIS measurements. All measurements were taken in a two-electrode system using open circuit voltage (VOC) of the DSSC as the biased voltage at the frequency range between 10−2 and 106 Hz under the illumination of 100 mWcm−2.
As illustrated in the Fig. 8a, series resistance (\({\text{R}}_{\text{s}}\)) was obtained from the lower intersection point between the small semicircle and the horizontal axis of the Nyquist plot which contributed to the sheet resistance of FTO. The semicircle appears in the high-frequency region is responsible for the charge transfer resistance at the counter electrode/electrolyte interface (\({\text{R}1}_{\text{c}\text{t}}\)) [38]. No significant changes were observed in the \({\text{R}}_{\text{s}}\) and \({\text{R}1}_{\text{c}\text{t}}\) values due to Ni doping. However, the charge transfer resistance of the TiO2/dye/electrolyte interface (\({\text{R}2}_{\text{c}\text{t}}\)) of the 0.10 wt% Ni-doped TiO2 based DSSC was found to be 13.0 Ω which was lower than that of the un-doped TiO2 based device (19.0 Ω) and led to improved charge transport at the TiO2/dye/electrolyte interface. This is another reason for the increased JSC values and enhancement in the PCEs of the Ni-doped TiO2 based devices. Moreover, 1.00 wt% Ni-doped TiO2 based device exhibited an increased \({\text{R}2}_{\text{c}\text{t}}\) value of 22.2 Ω which indicates that higher Ni doping causes random transit flow of electrons due to the formation of intermediate trap sites. As a result of this random transition motion of electrons, charge recombination increases [19, 20]. The Bode phase plots derived from EIS measurements on DSSCs are shown in Fig. 9b for the un-doped TiO2, 0.10 and 1.00 wt% Ni-doped TiO2 photoanodes. The electron life time (\({\varvec{T}}_{\varvec{e}}\)) was estimated using the following equation:
where \({f}_{max}\) is the maximum frequency corresponding to the peak [39, 40]. The measured electron lifetimes of the DSSCs with un-doped TiO2, 0.10 and 1.00 wt% Ni-doped TiO2 were found to be 7.1, 9.3 and 5.1 ms respectively (Table 4). It was noted that the electron lifetime of the DSSC with 0.1 wt% Ni-doped TiO2 was higher than the device with un-doped TiO2. The prolonged electron life times of the optimized 0.1 wt% Ni-doped TiO2 based device indicates suppression of the recombination rate. This is in consistent with the previously reported study by Balakrishnan and his group [21]. It should be noted that higher Ni-doping (1.00 wt%) reduces the electron lifetime due to formation of intermediate trap sites which leads to increased charge recombination.
4 Conclusion
In this study, Ni-doped TiO2 nanomaterials, with systematically varied Ni content from 0.05 to 1.00 wt%, were successfully synthesized by a facile impregnation method and the synthesized nanomaterials were characterized. Upon successful doping of Ni on TiO2, anatase and rutile phases of TiO2 were found to be retained; wavelength of light absorption showed a red shift due to reduced band gap; and dye adsorption enhanced due to increased surface area. Among the fabricated DSSCs, the device with 0.10 wt% Ni-doped TiO2 electrode showed optimum efficiency of 6.29% with over 20% enhancement compared to the control device under air mass (AM) 1.5 conditions (100 mWcm−2, 1 sun). This improvement in the PCE is due to the increased short circuit current density as a result of enhanced visible light harvest and improved charge transport in the device.
Data availability
All data generated or analysed during this study are included in this published article.
References
S. Borbón, S. Lugo, D. Pourjafari, N. Pineda Aguilar, G. Oskam, I. López, ACS omega 5, 10977 (2020)
S. James, R. Contractor, Sci. Rep. 8, 1 (2018)
S.M. Gupta, M. Tripathi, Cent. Eur. J. Chem. 10, 279 (2012)
K. Prashanthan, T. Thivakarasarma, P. Ravirajan, M. Planells, N. Robertson, J. Nelson, J. Mater. Chem. C 5, 11758 (2017)
T. Rajaramanan, S. Shanmugaratnam, V. Gurunanthanan, S. Yohi, D. Velauthapillai, P. Ravirajan, M. Senthilnanthanan, Catalysts 11, 690 (2021)
A. Pirashanthan, T. Murugathas, K. Mariappan, P. Ravirajan, D. Velauthapillai, S. Yohi, Mater. Lett. 274, 127997 (2020)
S. Loheeswaran, M. Thanihaichelvan, P. Ravirajan, J. Nelson, J. Mater. Sci. : Mater. Electron. 28, 4732 (2017)
S. Loheeswaran, K. Balashangar, J. Jevirshan, P. Ravirajan, J. Nanoelectron Optoelectron. 8, 484 (2013)
M.E. Yeoh, K.Y. Chan, Int. J. Energy Res. 41, 2446 (2017)
Y. Zhang, L. Wang, B. Liu, J. Zhai, H. Fan, D. Wang, Y. Lin, T. Xie, Electrochim. Acta 56, 6517 (2011)
A.K. Gupta, P. Srivastava, L. Bahadur, Appl. Phys. A 122, 1 (2016)
X. Lü, X. Mou, J. Wu, D. Zhang, L. Zhang, F. Huang, F. Xu, S. Huang, Adv. Funct. Mater. 20, 509 (2010)
X. Zhang, F. Liu, Q.-L. Huang, G. Zhou, Z.-S. Wang, J. Phys. Chem. C 115, 12665 (2011)
K. Sahu, M. Dhonde, V.V.S. Murty, Int. J. Energy Res. 45, 5423 (2021)
Y. Wang, R. Zhang, J. Li, L. Li, S. Lin, Nanoscale Res. Lett. 9, 1 (2014)
B. Roose, S. Pathak, U. Steiner, Chem. Soc. Rev. 44, 8326 (2015)
L.-L. Lai, W. Wen, J.-M. Wu, RSC Adv. 6, 25511 (2016)
Y.-M. Lin, Z.-Y. Jiang, C.-Y. Zhu, X.-Y. Hu, X.-D. Zhang, J. Fan, Mater. Chem. Phys. 133, 746 (2012)
T. Sakthivel, K.A. Kumar, J. Senthilselvan, K. Jagannathan, J. Mater. Sci. : Mater. Electron. 29, 2228 (2018)
T. Raguram, K. Rajni, J. Mater. Sci. : Mater. Electron. 32, 18264 (2021)
M. Balakrishnan, R. John, J. Mater. Sci. : Mater. Electron. 32, 5295 (2021)
A. Malik, S. Hameed, M. Siddiqui, M. Haque, K. Umar, A. Khan, M. Muneer, J. Mater. Eng. Perform. 23, 3184 (2014)
T. Rajaramanan, M. Natarajan, P. Ravirajan, M. Senthilnanthanan, D. Velauthapillai, Energies 13, 1532 (2020)
T. Karthik, R. Rathinamoorthy, R. Murugan, J. Ind. Text. 42, 99 (2012)
S.R. Miditana, T.S. Rao, S.A. Alim, J. Nanosci. Technol. 5, 682 (2019)
Z. Zhou, M. Li, L. Guo, J. Phys. Chem. Solids 71, 1707 (2010)
M. Alijani, N.N. Ilkhechi, Silicon 10, 2569 (2018)
T. Rajaramanan, F. Heidari Gourji, D. Velauthapillai, P. Ravirajan, M. Senthilnanthanan, Int. J. Energy Res. 202, 1090174 (2023)
Z. Li, D. Ding, Q. Liu, C. Ning, X. Wang, Nanoscale Res. Lett. 9, 118 (2014)
S. Varnagiris, M. Urbonavičius, S. Sakalauskaitė, E. Demikyte, S. Tuckute, Catalysts 11, 1454 (2021)
Z. Yao, F. Jia, S. Tian, C. Li, Z. Jiang, X. Bai, ACS Appl. Mater. Interfaces 2, 2617 (2010)
I. Ganesh, A. Gupta, P. Kumar, P. Sekhar, K. Radha, G. Padmanabham, G. Sundararajan, Sci. World J. 2012, 127326 (2012)
J. Manju, S.M.J. Jawhar, J. Mater. Res. 33, 1534 (2018)
S. Prathapani, V. More, S. Bohm, P. Bhargava, A. Yella, S. Mallick, Appl. Mater. Today 7, 112 (2017)
P. Archana, E.N. Kumar, C. Vijila, S. Ramakrishna, M. Yusoff, R. Jose, Dalton Trans. 42, 1024 (2013)
M.R. Elahifard, S. Ahmadvand, A. Mirzanejad, Mater. Sci. Semicond. Process. 84, 10 (2018)
P. Soni, V. Murty, K. Kushwaha, J. Nanosci. Nanoeng Appl. 8, 69 (2018)
H.F. Mehnane et al, RSC Adv. 7, 2358 (2017)
A. Ramar, R. Saraswathi, M. Rajkumar, S.-M. Chen, J. Phys. Chem. C 119, 23830 (2015)
T. Nikolay, L. Larina, O. Shevaleevskiy, B.T. Ahn, in 2011 37th IEEE Photovoltaic Specialists Conference (IEEE, 2011), pp. 000748
Acknowledgements
The authors express gratitude to the Capacity Building and Establishment of Research Consortium (CBERC) project, Grant Number LKA-3182-HRNCET and the Higher education and Research collaboration on Nanomaterials for Clean Energy Technologies (HRNCET) project, Grant number NORPART/2016/10,237 for financial support.
Funding
Open access funding provided by Western Norway University Of Applied Sciences. This research was funded by the Capacity Building and Establishment of a Research Consortium (CBERC) Project (grant number LKA-3182- HRNCET) and Higher Education and Research collaboration on Nanomaterials for Clean Energy Technologies (HRNCET) Project (grant number NORPART/2016/10237).
Author information
Authors and Affiliations
Contributions
TR: Conceptualization, Methodology, Data curation, Software, Investigation, Validation, Writing—Original draft preparation, Writing—Reviewing and Editing. DV: Data curation, Investigation, Validation, Supervision, Funding acquisition, Writing—Reviewing and Editing. PR: Data curation, Investigation, Validation, Supervision, Funding acquisition, Writing—Reviewing and Editing. MS: Data curation, Investigation, Validation, Supervision, Visualization, Writing—Reviewing and Editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajaramanan, T., Velauthapillai, D., Ravirajan, P. et al. A facile impregnation synthesis of Ni-doped TiO2 nanomaterials for dye-sensitized solar cells. J Mater Sci: Mater Electron 34, 916 (2023). https://doi.org/10.1007/s10854-023-10347-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10347-4