Abstract
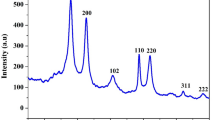
In the current research work, an electrochemically polymerized glutamine-clustered graphite paste electrode surface was developed for the electrochemical detection of hazardous catechol (CL) molecules. The cyclic voltammetric (CV) method was operated for the detection of redox activities of CL in phosphate buffer solution (PBS) of pH 7.0 on the surface of polymerised glutamine (GN) modified graphite (GP) paste electrode (P(GN)MGPPE). The electrochemical impedance (EI), field emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDXS), and CV methods were used to elucidate the surface behaviours and morphology of the prepared electrode materials. The P(GN)MGPPE provides a well-defined and highly sensitive electrochemical redox peak for CL based on an adsorption-controlled reaction approach compared to the bare graphite paste electrode (BGPPE) surface. The activated electrochemical sensor provides good linear relation among the concentrations, and the corresponding reduction peak currents of CL ranging from 2.0 to 200.0 μM and the calculated detection limit (DL) and quantification limit (QL) are found to be 0.045 µM and 0.151 µM, respectively. The activated electrode surface reveals high electrocatalytic activity with good reproducibility, repeatability, and stability. Finally, the P(GN)MGPPE surface was operated for the detection of CL in water samples with a fine recovery ranging from 99.23 to 100.42%.










Similar content being viewed by others
Data availability
The data underlying this article are presented in the main manuscript. The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
L.M. Sousa, L.M. Vilarinho, G.H. Ribeiro, A.L. Bogado, L.R. Dinelli, An electronic device based on gold nanoparticles and tetraruthenatedporphyrin as an electrochemical sensor for catechol. R Soc Open Sci. 20, 170675 (2017)
U.D.O. Health, H. Services, Hazardous substances data bank (HSDB, online database), National Toxicology Information Program, National Library of Medicine, Bethesda, MD, (1993).
M.J. Schoning, M. Jacobs, A. Muck, D.T. Knobbe, J. Wang, M. Chatrathi, S. Spillmann, Amperometric PDMS/glass capillary electrophoresis-based biosensor microchip for catechol and dopamine detection. Sens. Actuators B Chem. 108, 688–694 (2005)
S. Timur, N. Pazarlioglu, R. Pilloton, A. Telefoncu, Detection of phenolic compounds by thick film sensors based on Pseudomonas putida. Talanta 61, 87–93 (2003)
Z. Zhang, J. Liu, J. Fan, Z. Wang, L. Li, Detection of catechol using an electrochemical biosensor based on engineered Escherichia coli cells that surface-display laccase. Anal Chim Acta. 7, 65–72 (2018)
V. Sethuraman, P. Muthuraja, J. Anandha Raj, P. Manisankar, A highly sensitive electrochemical biosensor for catechol using conducting polymer reduced graphene oxide–metal oxide enzyme modified electrode. Biosens. Bioelectron. 84, 112–119 (2016)
L. Fan, X. Li, X. Kan, Disposable graphite paper-based sensor for sensitive simultaneous determination of hydroquinone and catechol. Electrochim. Acta. 213, 504–511 (2016)
M.M. Rao, R. Settu, S.M. Chen, P. Alagarsamy, T.W. Chen, I.S. Hong, Electrochemical determination of catechol using functionalized multiwalled carbon nanotubes modified screen printed carbon electrode. Int. J. Electrochem. Sci. 13, 6126–6134 (2018)
E. Lebègue, R.O. Louro, F. Barrière, Electrochemical detection of pH-responsive grafted catechol and immobilized cytochrome c onto lipid deposit-modified glassy carbon surface. ACS Omega 3, 9035–9042 (2018)
G. Marrubini, E. Calleri, T. Coccini, A. Castoldi, L. Manzo, Direct analysis of phenol, catechol and hydroquinone in human urine by coupled-column HPLC with fluorimetric detection. Chromatographia 62, 25–31 (2005)
Y. Hu, X. Li, Z. Pang, Indirect chemiluminescence detection for capillary zone electrophoresis of monoamines and catechol using luminol-K3[Fe(CN)6] system. J. Chromatogr. A 1091, 194–198 (2005)
E.C. Figueiredo, C.R.T. Tarley, L.T. Kubota, S. Rath, M.A.Z. Arruda, On-line molecularly imprinted solid phase extraction for the selective spectrophotometric determination of catechol. Microchem. J. 85, 290–296 (2007)
E. Lourenco, A. Ferreira, E. Pinto, M. Yonamine, S. Farsky, On-fiber derivatization of SPME extracts of phenol, hydroquinone and catechol with GC-MS detection. Chromatographia 63, 175 (2006)
L. Zhao, B. Lv, H. Yuan, Z. Zhou, D. Xiao, A sensitive chemiluminescence method for determination of hydroquinone and catechol. Sensors 7, 578–588 (2007)
X. Yuan, B. Wang, C. Yan, W. Lv, Q. Ma, B. Zheng, J. Du, D. Xiao, A rapid and simple strategy for discrimination and detection of catechol and hydroquinone by fluorescent silicon nanoparticles. Microchem. J. 158, 105263 (2020)
C. Liu, J. Hu, S. Biswas, F. Zhu, J. Zhan, G. Wang, C.H. Tung, Y. Wang, Surfaceenhanced Raman scattering of phenols and catechols by a molecular analogue of titanium dioxide. Anal. Chem. 92, 5929–5936 (2020)
F. Keshvari, M. Bahram, Selective, sensitive and reliable colorimetric sensor for catechol detection based on anti-aggregation of unmodified gold nanoparticles utilizing boronic acid–diol reaction: optimization by experimental design methodology. J. Iran. Chem. Soc. 14, 977–984 (2017)
N.S. Prinith, J.G. Manjunatha, A.A. Al-Kahtani, A.M. Tighezza, M. Sillanpää, Highly selective and sensitive voltammetric method for the detection of catechol in tea and water samples using poly(gibberellic acid)-modified carbon paste electrode. ACS Omega 7, 24679–24687 (2022)
F. Mollarasouli, S. Kurbanoglu, K. Asadpour-Zeynali, S.A. Ozkan, Preparation of porous Cu metal organic framework/ZnTe nanorods/Au nanoparticles hybrid platform for nonenzymatic determination of catechol. J. Electroanal. Chem. 856, 113672 (2020)
J.G. Manjunatha, Poly (Adenine) modified graphene-based voltammetric sensor for the electrochemical determination of catechol, hydroquinone and resorcinol. Open Chem. Eng. J. 14, 52–62 (2020)
S. Nikhil, A. Karthika, P. Suresh, A. Suganthi, M. Rajarajan, A selective and sensitive electrochemical determination of catechol based on reduced graphene oxide decorated β-cyclodextrin nanosheet modified glassy carbon electrode. Adv. Powder Technol. 32, 2148–2159 (2021)
J.G. Manjunatha, A promising enhanced polymer modified voltammetric sensor for the quantification of catechol and phloroglucinol. Anal. Bioanal. Electrochem. 12, 893–903 (2020)
N. Hareesha, J.G. Manjunatha, A simple and low-cost poly(dl-phenylalanine) modified carbon sensor for the improved electrochemical analysis of Riboflavin. J. Sci.: Adv Mater. Devices 5, 502–511 (2020)
J.G. Manjunatha, A surfactant enhanced graphene paste electrode as an effective electrochemical sensor for the sensitive and simultaneous determination of catechol and resorcinol. Chem. Data Collect. 25, 100331 (2020)
N. Hareesha, J.G. Manjunatha, B.M. Amrutha, N. Sreeharsha, S.M. Basheeruddin Asdaq, K. Anwer, A fast and selective electrochemical detection of vanillin in food samples on the surface of poly(glutamic acid) functionalized multiwalled carbon nanotubes and graphite composite paste sensor. Coll. Surf. A Physicochem. Eng. Asp. 626, 127042 (2021)
N. Hareesha, J.G. Manjunatha, B.M. Amrutha, P.A. Pushpanjali, M.M. Charithra, N. Prinith Subbaiah, Electrochemical analysis of indigo carmine in food and water samples using a poly(glutamic acid) layered multi-walled carbon nanotube paste electrode. J. Electron. Mater. 50, 1230–1238 (2021)
J.M. Lacey, D.W. Wilmore, Is glutamine a conditionally essential amino acid?". Nutr. Rev. 48, 297–309 (1990)
Y. Zhu, S. Huai, J. Jiao, Q. Xu, H. Wu, H. Zhang, Fullerene and platinum composite based electrochemical sensor for the selective determination of catechol and hydroquinone. J. Electroanal. Chem. 878, 114726 (2020)
M. Coros, F. Pogacean, L. Magerusan, M.C. Rosu, A.S. Porav, C. Socaci, A. Bende, R.I. Stefan-van Staden, S. Pruneanu, Graphene-porphyrin composite synthesis through graphite exfoliation: the electrochemical sensing of catechol. Sens. Actuators B Chem. 256, 665–673 (2018)
W. Si, W. Lei, Y. Zhang, M. Xia, F. Wang, Q. Hao, Electrodeposition of graphene oxide doped poly (3, 4-ethylenedioxythiophene) film and its electrochemical sensing of catechol and hydroquinone. Electrochim. Acta 85, 295–301 (2012)
L. Zhao, J. Yu, S. Yue, L. Zhang, Z. Wang, P. Guo, Q. Liu, Nickel oxide/carbon nanotube nanocomposites prepared by atomic layer deposition for electrochemical sensing of hydroquinone and catechol. J. Electroanal. Chem. 808, 245 (2018)
Y. Xin, N. Wang, C. Wang, W. Gao, M. Chen, N. Liu, J. Duan, B. Hou, Electrochemical detection of hydroquinone and catechol with covalent organic framework modified carbon paste electrode. J. Electroanal. Chem. 877, 114530 (2020)
Funding
N. Hareesha is grateful for the financial support from the Department of Science and Technology, Govt. of India for the INSPIRE Fellowship (Registration number: IF180479). Ammar M. TIGHEZZA and Munirah D. Albaqami are grateful for the Researchers Supporting Project Number (RSP2023R267) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
NH: Conceptualization, Formal analysis, Methodology, Software, Data curation, Visualization, Writing—original draft, Writing—review & editing. JGM: Conceptualization, Supervision, Formal analysis, Methodology, Data curation, Visualization, Writing—review & editing. CR: Formal analysis, Methodology, and Data curation. AMT: Formal analysis and Data curation. MDA: Formal analysis and Data curation. MS: Formal analysis and Data curation.
Corresponding author
Ethics declarations
Competing interest
We have no known potential competing financial interests or personal relationships that could have appeared to influence the reported work.
Ethical approval
This article is original and has not been published previously in any form or language, and this article is not under consideration for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hareesha, N., Manjunatha, J.G., Raril, C. et al. Electrochemically polymerized glutamine-activated graphite paste surface as a green biosensor for sensitive catechol detection in water samples. J Mater Sci: Mater Electron 34, 533 (2023). https://doi.org/10.1007/s10854-023-09951-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-09951-1