Abstract
The fabrication of diodes using organic semiconductors as active materials has gained wide interest, and has undergone significant progress in the past few decades. A naphthalimide-indole derivative (BNIA) was synthesized through bromination of acenaphthene, subsequent oxidation, and finally imidation with indole-3-acetic anhydride. The molecular structure was characterized using NMR and IR spectroscopy. The BNIA displayed good thermal stability up to 300 °C and an electrochemical band gap of 1.07 eV. The theoretical studies were performed to support the experimental findings. The electrical parameter values and the dielectric measurement studies directed towards the suitability of BNIA as a functional material in electronic devices. A diode was successfully constructed using BNIA as the n-type material with an ideality factor of 8.13, and the electrical parameters are analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The research developments in organic electronics are always driven by new and smart materials, improved and advanced engineering, as well as technology revolutions. The growing demand for materials that offer simpler and less-expensive processing methods to realize a better future has fuelled interest in the quest of organic semiconductors. Small molecules-based organic electronics is a thrust research arena that has attracted ample attention not only due to the successful fabrication of extremely conformable, flexible, and thin electronic devices, but also because of being more cost-effective and easy to process compared to the inorganic materials-based conventional devices [1, 2]. Thus, organic semiconductors have gained wide interest as an alternative to silicon-based inorganic semiconductors.
Organic semiconducting small molecules have striking prospects as active materials in flexible electronics as they benefit from being synthesized with good purity without any batch-wise variations, having a distinct molecular structure, definite molecular weight, easily functionalisable skeletal framework to attain desired features including HOMO and LUMO levels, absorption coefficient, charge carrier capacity etc. Hence, an appropriate modification of these functional molecules to realize desirable properties in various devices including non-linear optics, organic photovoltaics, organic field-effect transistors, and molecular sensors is of immense importance [2,3,4]. Intense research is still going on to achieve organic semiconductors that could circumvent the challenges such as good thermal stability and diverse fabrication techniques for better devices [5,6,7]. Molecular geometry, electronic features and intermolecular charge transport capability between the adjacent π-conjugated species are the main factors that govern the semiconducting properties of organic small molecules [8,9,10,11]. Thus, a rational molecular design could possibly result in the development of organic semiconductors with superior performance for suitable application in organic electronics with real-life commercial application [12].
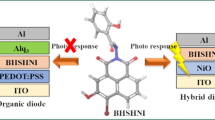
In the recent past, 1,8-naphthalimide (NI) derivatives with electron-deficient, planar, and rigid skeletal framework have garnered increased attention of material researchers due to their attractive optical, electrochemical and photoelectrical properties [13]. These molecules have been widely used in diverse applications including photoinduced electron transfer sensors, fluorescent biological markers, fluorescence switchers, ionic probes [14, 15] etc. The NI derivatives can exhibit relatively greater electron-affinity, low reduction potentials [16,17,18] and hence wide energy gaps [19,20,21]. Moreover, they also own good charge transfer property, and better film forming ability. Therefore, the NI-based structures are studied profoundly as electron-deficient n-type organic semiconductors [22, 23]. Besides, there are literature evidences that suggest the use of indole based p-type hole transport materials with high glass transition temperature in OLEDs [24]. Though there are many reports on NI derivatives in OLEDs, there are no investigations on the construction of electrical circuit elements using an organic structural framework that combines both indole and NI. Hence, this study is an attempt to synthesize a NI-indole conjugate, and examine the intrinsic features of the new small molecule. Further, it is used as an active material to fabricate a diode and analyse the device features.
2 Materials and methods
The solvents and chemicals for the synthesis of NI-indole derivative were procured from Sigma, and Spectrochem Chemicals Pvt. Ltd. The open capillary method was used to record the melting point, and is uncorrected. The IR and NMR spectra were obtained using Shimadzu FTIR spectrophotometer and 400 MHz Bruker spectrometer, respectively. Elemental analysis was performed using varioMICRO select analyser. Mettler Toledo DSC-3 and HITACHI thermal analyser STA7000 series were used to record the Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) thermograms at a heating rate of 10 °C min−1, respectively under nitrogen atmosphere. The Carl Zeiss EVO 18 analytical Scanning Electron Microscope (SEM) and INNOVA SPM Atomic Force Microscope (AFM) were used to inspect the surface morphology of the thin film. The electrochemical measurements were performed at 25 °C using a CHI660D potentiostat instrument in a one compartment N2-saturated three-electrode cell; where the working, reference and counter electrodes were glassy carbon (polished with 1.0 μm alumina slurry and sonicated in distilled water for 10 min), Ag/Ag+ and platinum, respectively. The uniform thin film of NI-indole conjugate obtained on the glassy carbon disk using drop casting (1 mg of BNIA in 1 mL ethanol) method was dipped into 0.1 M tetrabutylammonium hexafluorophosphate in 10 mL acetonitrile solution. The Cyclic Voltammetry (CV) studies were carried out at a scan rate of 100 mV/s. Ferrocene/ ferrocenium (Fc/Fc+) redox couple was used as an external standard to calibrate the Ag/Ag+ reference electrode. The impedance studies and Current-Voltage (I–V) measurements of the diode were performed using Hioki LCR meter IM 3635 and Keithley 2400 source meter, respectively.
3 Results and discussion
3.1 Synthesis of BNIA
Acenaphthene was brominated and the product was further oxidized to obtain 4-bromo-1,8- naphthalic anhydride. The anhydride was allowed to undergo imidation reaction with indole-3-acetic hydrazide to afford the new NI-indole conjugate, BNIA. The synthesis route is portrayed in Scheme 1.
N-(6-bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-2-(1H-indol-3-yl)acetamide (BNIA).
4-Bromo-1,8-naphthalic anhydride (1 mmol) and indole-3-acetic hydrazide (1 mmol) were dissolved in ethanol (5 mL each) and refluxed for 12 h. The yellow precipitate obtained was dried and recrystalized using ethanol to obtain pure BNIA.
3.2 Structural characterization of BNIA
N-(6-bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-2-(1H-indol-3-yl)acetamide: yellow crystals (yield: 45%), m.p- 236–238 °C, FT-IR (ATR, cm−1) (Fig. S1): 3312 (NH str.), 3051 (Ar. CH str.), 1722, 1695, 1671 (C=O str.), 1236 (C–N str.), 734 (C–Br str.); 1H NMR (DMSO-d6, 400 MHz) (Fig. S2): δ 3.821 (s, 2H, CH2), 7.004–7.041 (t, 1H, 7.2 Hz), 7.082–7.118 (t, 1H, 7.2 Hz), 7.360–7.382 (d, 2H, 8.8 Hz), 7.678–7.697 (d, 1H, 7.6 Hz), 8.032–8.072 (t, 1H, 8 Hz), 8.269–8.288 (d, 1H, 7.6 Hz), 8.391–8.411 (d, 1H, 8 Hz), 8.631–8.649 (d, 2H, 7.2 Hz), 10.939 and 10.949 (s, NH, 2H); 13C NMR (DMSO-d6, 100 MHz) (Fig. S3): δ 30.82, 108.13, 111.77, 118.87, 119.24, 121.51, 122.20, 122.98, 124.47, 127.71, 128.67, 129.55, 130.51, 130.56, 132.13, 132.17, 132.87, 133.93, 136.57, 161.63, 161.67, 170.05; Elemental analysis for C22H14BrN3O3: calculated: C-58.94, H-3.14, N-9.37; found: C-58.73, H-3.16, N-9.34.
3.3 UV-Visible spectroscopy
UV spectrum of BNIA in THF (1 × 10−4 M) depicted absorption peaks around 280 and 350 nm as presented in Fig. 1A, which could be due to π–π* and n–π* transitions [25, 26]. The optical band gap for the NI-indole derivative was calculated using tauc plot (Fig. S4) and was found to be 3.12 eV.
3.4 Electrochemical studies
The molecular orbital energy levels of a material are connected to the redox potentials, and determine the electron/hole-injecting capability in an electronic device. The reduction and oxidation onset potentials for BNIA were extracted from the cyclic voltammogram (Fig. 1B), and were 0.46 eV and 1.53 eV, respectively. Further, the HOMO and the LUMO levels determined using CV measurements were found to be − 5.83 eV and − 4.76 eV, respectively, with an electrochemical band gap of 1.07 eV.
3.5 Thermal analysis
For potential use in electronic devices, it is critical to ensure that the synthesized molecule can withstand high temperature ranges during its operation. DSC and TGA analysis were performed to understand the thermal stability of BNIA. The DSC thermogram (Fig. 1C) exhibited a sharp endothermic peak at 243 due to its melting, which was consistent with the result acquired utilising the open capillary technique. Furthermore, the TGA thermogram (Fig. 1D) revealed no other heat changes, indicating its ability to withstand temperatures of up to 300 °C.
3.6 Surface morphology analysis
Uniform film forming property of BNIA was probed by examining the surface morphology using SEM and AFM. The SEM image of BNIA thin film obtained through physical vapor deposition technique displayed tiny globular surfaces as noticed in Fig. 2A. The surface roughness of the film measured using AFM technique (Fig. 2B) was found to be 3.84 nm (Rq). Minimal roughness of surface indicates uniform compound deposition, and hence suggests that BNIA can be coated uniformly during the fabrication of an electronic device.
3.7 Theoretical studies
To develop the optimal molecular geometry, and subsequently analyze the electronic density arrangement in the HOMO and LUMO levels of BNIA, simulations were accomplished using the Schrodinger software tool using B3LYP basic set. A stable minimum energy conformation was obtained along with molecular orbital energy levels as observed in Fig. 2C. The HOMO, LUMO energy levels and the band gap were calculated to be − 5.504 eV, − 3.273 eV and 2.231 eV, respectively.
3.8 Electrical characterization
The intrinsic charge transport mechanisms, charge carrier concentrations, mobility and resistivity of BNIA was elucidated using Hall Effect measurements. The NI-indole derivative was found to be a n-type semiconductor with high carrier concentration of 5.73 × 1016 cm−3 and 20.67 cm2/Vs mobility, which are useful for application in electronic devices. Moreover, the dielectric studies were accomplished in the frequency range of 10 to 5000 Hz by depositing BNIA between ITO coated plate and aluminium contacts through thermal evaporation under 6 × 10−6 mbar vacuum, which functioned as a parallel plate capacitor. The frequency dependent capacitance was measured and the plot presented in Fig. 3A indicates a higher capacitance value at a lower frequency, which drops down suddenly to attain a constant value. This trend in the capacitance value could be attributed to the majority and minority carriers as well as the interfacial states, which followed the AC current contributing to the capacitance at lower frequency. However, at higher frequency, the minority carriers and a part of the interface states cannot follow AC current, and thereby showed a relatively lower capacitance value [27, 28]. The capacitance value recorded for BNIA was ~ 0.013 nF at higher frequencies. The ability of a material to store electric flux and act as a capacitor is measured by its dielectric constant. Therefore, the frequency dependent dielectric constant of BNIA was determined and plotted against frequency as shown in Fig. 3B. The dielectric constant for BNIA was found to be < 1 at higher frequencies, which might be due to higher conductance. The higher dielectric constant obtained at lower frequency might be attributed to the polarization of BNIA, but sudden decrease in the dielectric constant could be due to the reduction of space charge polarisation effect [29,30,31]. The low dielectric loss as indicated in Fig. 3C indicates the minimum randomness in the system. Further, the frequency-dependent AC conductivities that depict the amount of electric current flowing through BNIA was calculated. The AC conductivity value obtained for BNIA from Fig. 3D was ~ 3.11 nΩ−1 cm−1 at higher frequencies, and is found to abruptly increase at higher frequencies. This result could be attributed to the rise in the series resistance of the dipole–dipole interactions [32].
The summary of the important material parameters obtained through the experimental and theoretical studies for BNIA are listed in Table 1. The values of the optical and electrochemical bandgaps of BNIA vary because it is well established that the electrochemical studies demonstrate definite HOMO, LUMO levels and bandgap, but the optical bandgap is dependent on several factors such as grain size and shape, and in turn the molarity of the precursor [33]. The difference in the theoretical and experimental band gap values is attributed to the measurement of theoretical values in perfect or near-perfect conditions by the software.
3.9 Diode characterization
A p-n diode having ITO | PEDOT:PSS | BNIA | Alq3 | Al as device configuration was constructed. On pre-coated ITO plates, PEDOT:PSS was spin coated, 100 nm BNIA and 60 nm Alq3 were thermal evaporated (6 × 10−6 mbar vacuum and 0.1–0.5 Å/s evaporation rate). Aluminium (Al: 100 nm) was coated on top of Alq3 by thermal evaporation at 1–5 Å/s. In the device, ITO functioned as anode, PEDOT:PSS as the hole transport material, BNIA as the n-type semiconductor, Alq3 as the electron transport layer and Al as the cathode. The I–V graphs (Fig. 4A) were recorded for the device, and current in micro ampere was observed. The ideality factor defines how closely the ideal diode equation is followed by the constructed diode. The value is 1 for an ideal diode, whereas it can be > 1 for real diodes. The ideality factor calculated for the device using log I vs. V plot (Fig. 4B) was found to be 8.14.
As the fabricated diode can act as a parallel plate capacitor, dielectric measurements were performed from 10 to 2000 Hz. The capacitance vs. frequency plot of the device with BNIA as active material is shown in Fig. 4C, and the capacitance value at 2000 Hz was 0.05 nF. Frequency dependent dielectric constant plot (Fig. 4D) exhibited a reduction in the dielectric constant, which might be due to the failure of the interfacial states and minor charges to respond to the AC current. The dielectric constant of the device was 0.85 at 1000 Hz. As shown in Fig. 4E, dielectric loss was perceived to be minimal in the system. Further, from the frequency-dependent AC conductivity plot as depicted in Fig. 4F, the AC conductivity at higher frequency for the device was found to be ~ 11 nΩ−1 cm−1.
4 Conclusion
The present work is a contribution towards efficient diodes that can be used in organic electronic circuits. The naphthalimide-indole derivative, BNIA was synthesized using simple methods and characterised using IR and NMR spectroscopy. BNIA was found to be stable till 300 °C and displayed an electrochemical bandgap of 1.07 eV. Electrical studies revealed a high carrier concentration of 5.73 × 1016 cm−3 with 20.67 cm2/Vs mobility for BNIA, which acts as a n-type material. A successful diode was constructed using BNIA, which exhibited an ideality factor of 8.13. The material BNIA can be further explored for its potential use in organic electronics.
Data availability
The datasets generated during and/or analyzed during the current study are available from the first author upon reasonable request.
References
M. Eslamian, Nano-Micro Lett. 9, 1 (2017)
W. Zhao, Z. He, J.W.Y. Lam, Q. Peng, H. Ma, Z. Shuai, G. Bai, J. Hao, B.Z. Tang, Chem. 1, 592 (2016)
G.B. Bodedla, K.R.J. Thomas, M. Fan, K. Ho, J. Org. Chem. 81, 640 (2015)
J.C. Furgal, J.H. Jung, T.G. Goodson, R.M. Laine, J. Am. Chem. Soc. 135, 12259 (2013)
A. Kukhta, E. Kolesnik, I. Grabchev, S. Sali, J. Fluoresc. 16, 375 (2006)
G. Ding, Z. Xu, G. Zhong, S. Jing, F. Li, W. Zhu, Res. Chem. Intermed. 34, 299 (2008)
J. Liu, J. Cao, S. Shao, Z. Xie, Y. Cheng, Y. Geng, L. Wang, X. Jing, F. Wang, J. Mater. Chem. 18, 1659 (2008)
V. Coropceanu, A. Demetrio, S. Filho, Y. Olivier, R. Silbey, J. Bre, Chem. Rev. 107, 926 (2007)
C. Liu, K. Huang, W.T. Park, M. Li, T. Yang, X. Liu, L. Liang, T. Minari, Y.Y. Noh, Mater. Horizons 4, 608 (2017)
G. Gryn’Ova, K.H. Lin, C. Corminboeuf, J. Am. Chem. Soc. 140, 16370 (2018)
K.A. Mcgarry, W. Xie, C. Sutton, C. Risko, Y. Wu, V.G. Young, Chem. Mater. 25, 2254 (2013)
H. Zhang, X. Wang, L. Yang, S. Zhang, Y. Zhang, C. He, W. Ma, J. Hou, Adv. Mater. 29, 1 (2017)
S. Chen, P. Zeng, X. Wang, Y. Wu, P. Lin, Z. Peng, J. Mater. Chem. C 7, 2886 (2019)
E. Martin, R. Weigand, A. Pardo, J. Lumin. 68, 157 (1996)
W.W. Stewart, J. Am. Chem. Soc. 103, 7616 (1981)
W. Jiang, Y. Sun, X. Wang, Q. Wang, W. Xu, Dye. Pigment. 77, 125 (2008)
P. Xiao, F. Dumur, B. Graff, D. Gigmes, J.P. Fouassier, J. Lalevée, Macromolecules 47, 601 (2014)
D. Kolosov, V. Adamovich, P. Djurovich, M.E. Thompson, J. Am. Chem. Soc. 124, 9945 (2002)
J. Zhang, H. Xiao, X. Zhang, Y. Wu, G. Li, C. Li, X. Chen, W. Ma, Z. Bo, J. Mater. Chem. C 4, 5656 (2016)
S. Saha, A. Samanta, J. Phys. Chem. A 106, 4763 (2002)
D.M. Han, H.J. Song, C.H. Han, Y.S. Kim, RSC Adv. 5, 32588 (2015)
Y. Wang, X. Zhang, B. Han, J. Peng, S. Hou, Y. Huang, H. Sun, M. Xie, Z. Lu, Dye. Pigment. 86, 190 (2010)
N.V. Marinova, N.I. Georgiev, V.B. Bojinov, J. Photochem. Photobiol A Chem. 254, 54 (2013)
M.S. Park, J.Y. Lee, Thin Solid Films 548, 603 (2013)
Y. Xue, J. Mou, Y. Liu, X. Gong, Y. Yang, L. An, Cent. Eur. J. Chem. 8, 928 (2010)
Y. Xue, X. Gong, J. Mol. Struct. THEOCHEM 901, 226 (2009)
P. Chattopadhyay, B. RayChaudhuri, Solid State Electron. 36, 605 (1993)
Y. Lee, J. Lee, J. Park, Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A 370, 73 (2001)
F.M. Pontes, E.R. Leite, E. Longo, J.A. Varela, E.B. Araujo, J.A. Eiras, Appl. Phys. Lett. 76, 2433 (2000)
P. Zubko, D.J. Jung, J.F. Scott, J. Appl. Phys. 100, 114112 (2006)
S.K. Patil, M.Y. Koledintseva, R.W. Schwartz, W. Huebner, J. Appl. Phys. 104, 074108 (2008)
K.F. El-Nemr, M.R. Balboul, M.A. Ali, J. Thermoplast Compos. Mater. 29, 704 (2016)
S. Benramache, O. Belahssen, A. Guettaf, A. Arif, J. Semicond. 35, 042001–042004 (2014)
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The authors declare that no funds, Grants, or other support were received for this work.
Author information
Authors and Affiliations
Contributions
YM and SK Methodology, experimental work, prepared the draft manuscript. DS and DK: Conceptualization, Supervision, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
MJP, Y., Kagatikar, S., Sunil, D. et al. n-Type naphthalimide-indole derivative for electronic applications. J Mater Sci: Mater Electron 34, 334 (2023). https://doi.org/10.1007/s10854-023-09827-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-09827-4