Abstract
A series of Dy3+ and Eu3+ co-doped zinc aluminoborosilicate (ZABS) glasses were synthesized by a high-temperature melt-quenching method. Visible and NIR transitions of Dy3+-Eu3+ ions are observed through absorption spectra. A reverse trend in the optical band gap values and Urbach energy are seen with addition of Eu3+ ions. Photoluminescence studies recorded under different excitation wavelengths showed a variation in the emission intensities and prevailed the color tuneability behaviour of dopants. The energy transfer between Dy3+ and Eu3+ ions are studied through emission profiles, energy level diagram, and decay curves. The type of multipolar interaction between Dy3+ and Eu3+ are understood via Inokuti-Hirayama (IH) model and Dexter energy model. The CIE chromaticity coordinates, and correlated color temperature (CCT) values suggest that the prepared glasses can be used for light emitting diode application when excited at near-ultraviolet region.
Similar content being viewed by others
1 Introduction
White light emission from single light emitting components like phosphor played a significant role in the lighting industry due to its peculiar property of giving high brightness and color quality [1]. An attempt to obtain white light with tri-color-based phosphors fascinated researchers in recent times [2]. However, such a method resulted a variation in the color of phosphors as time goes on and needs different drive voltages for different color emitting components [3]. Therefore, in an urge to gain white light from an identical luminescent center via the combination of different color emissions, different methods were attempted. One of the most pivotal methods to obtain a single-phased white light with uplifted emission is through energy transfer between two rare earths i.e., a sensitizer and an activator such as Tb3+/Dy3+, Ce3+/Dy3+ [4, 5]. In common, Dy3+ ions are included as sensitizer in co-doped materials due to their intense emission bands in the blue (482 nm) and yellow (575 nm) regions corresponding to the transition levels of 4F9/2 → 6H15/2 and 4F9/2 → 6H15/2 [6]. Nevertheless, the lack of red-component in Dy3+ makes it unsuitable for practical applications [7]. Hence, an efficient red light emitting material should be combined with Dy3+ to give a stable white light. Among the lanthanides, Eu3+ is known to emit intense red light from the 5D0 → 7F1 and 5D0 → 7F2 levels falling in the visible region such as 590 nm and 613 nm [8]. Combining Dy3+ with Eu3+ will tend to enforce the europium emission, and thereby rectify the lack of red-component with singly doped Dy3+ ions. The yellow to blue emission intensity ratio (Y/B ratio) in Dy3+-based materials can be tuned with incorporation of Eu3+ ions in smaller intervals. When Dy3+ combined with Eu3+ materials are excited with n-UV light source the Dy3+ ions absorb the incident light, and they transfer the part of absorbed energy non-radiatively to Eu3+ ions causing emissions. Moreover, the white light obtained via co-doping can be adjusted or tuned from a cold white light to warm white light with increasing the Eu3+content [9]. Therefore, Dy3+ and Eu3+ co-doped materials have gained lots of interest in the past years.
Compared to rare earth doped phosphors [7, 8], glasses act as an efficient center due to their strong dispersion of RE (rare earth) ions in the glass matrix, better thermal stability, and sharp electronic spectra of RE ions with less crystal-field splitting. Many research articles reported the color tuneability, and energy transfer of Dy3+ and Eu3+ co-doped glasses with different glass matrices [11,12,13,14,15,16,17]. Among different host matrices, borosilicate glasses with two major component such as B2O3 and SiO2 are a potential choice since they own several exceptional properties like high thermal resistance, easy solubility of RE ions, good mechanical properties, and low thermal expansion coefficient [10]. Therefore, in the present work the color tuneability behaviour of co-doped zinc alumino borosilicate glasses, with varying Eu3+ concentrations, under different excitation wavelengths is analyzed. Also, the type of energy transfer interaction from the sensitizer (Dy3+) to the activator (Eu3+) are reported using Inokuti-Hirayama (IH) fitting and Dexter energy transfer model. The obtained results showed the suitability of prepared glasses for near-ultraviolet W-LEDs applications.
2 Experimental details
Transparent glasses with glass matrix formula given as 20SiO2–(20–x–y)B2O3–10Al2O3–10ZnO–30NaF–10ZnF2–xDy2O3–yEu2O3 (where x = 0.5 mol% and y = 0, 0.1, 0.5, 1.0, 1.5, 2.0 and 2.5 mol%) were synthesized using high-temperature melt-quenching method. The starting materials were initially taken by proper weighing of them to get a total of 10-gram quantity of glass. After a constant grinding of raw materials, they are melted in an alumina crucible at 1320 °C for 2 h. The formed melt is cascaded quickly on a pre-heated brass plate at 350 °C. Once the melt is released or quenched on the brass plate, it immediately forms a solid glass. The solid glass is further annealed for 2 h at the quenched temperature to reduce thermal stresses, avoid breaking of glass and to maintain transparency. The glasses were polished to get a smooth surface and their thicknesses was reduced to ~ 2 mm for optical studies. The prepared glasses were labelled as ZABSDE0, ZABSDE1, ZABSDE2, ZABSDE3, ZABSDE4, ZABSDE5 and ZABSDE6, respectively.
3 Result and discussion
3.1 UV-Visible-NIR studies
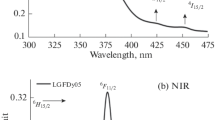
The absorption spectra recorded for the synthesized glasses showed several peaks comprising of both Dy3+ and Eu3+ ions. In the case of Dy3+, the absorbance excitation occurs from a single ground state at 6H15/2 while for Eu3+ there are two ground states of 7F0 and 7F1 such that the absorption transitions occur from both these levels. Figure 1(a) shows the UV-Visible part of the absorption spectra. Here the transitions peaks corresponding to Dy3+ ions are located at 350 (6P7/2), 364 (4I11/2 + 6P5/2), 387 (4I13/2 + 4F7/2), 425 (4G11/2), 452 (4I15/2), and 473 nm (4F9/2); whereas the transitions peaks of Eu3+ ions are observed at 362 (7F0→5D4), 376 (7F0→5L7), 383 (7F0→5G2), 393 (7F0→5L6), 414 (7F0→5D3), 464 (7F0→5D2), 525 (7F0→5D1), and 532 nm (7F1→5D1). The absorption intensity of the peaks is improved with co-doping of Eu3+ ions to Dy3+ ions [11, 12]. The Dy3+ peaks are seen for all co-doped (Dy3+-Eu3+) glasses except for the peaks present around 376 nm, 393 nm, 414 nm, 525 nm, and 532 nm which are purely due to Eu3+ ions [12]. For Dy3+ singly doped glass, the peaks observed at 364 and 387 nm gets slightly shifted to the lower wavelength side on addition of Eu3+ ions for co-doped glasses. Thus, the overlapping of energy levels of Dy and Eu in the ultraviolet region exist and are shown in yellow colour in the inset of Fig. 1. This overlapping of Dy3+ and Eu3+ energy levels signifies some sort of energy transfer behaviour existing in the glasses. In NIR region lying between 700 and 2500 nm (Fig. 1b), the Dy3+ ions transitions are seen at 752 (6F3/2), 801 (6F5/2), 899 (6F7/2 + 6H5/2), 1087 (6F9/2 + 6H7/2), 1267 (6F11/2 + 6H9/2), and 1687 nm (6H11/2) and the Eu3+ transitions are seen at 2090 nm (7F0→7F6) and 2203 nm (7F1→5F6). Increasing Eu3+ concertation in the glass also improves the absorbance intensity of Dy3+ and Eu3+ ions [16, 17].
Figure 2a represents the absorption band-edge plot which shows a red shift in the band-edges of the glasses with varying Eu3+ concentration. The optical band gap of the glasses was determined by drawing Tauc’s plot (Fig. 2b) using the following relation given as [18, 19].
In the Eq. (1), the terms \(\alpha , h\), \(\nu\), \({E}_{g}\) denote the absorption coefficient, Planck’s constant, photon frequency, and bandgap energy. The term \(B\) is a constant known as band-tailing parameter and \(n\) is the power factor that determines the nature of the electronic transition. A value of \(n=\frac{1}{2}\) shows a direct bandgap and while \(n=2\) indicates an indirect one. For amorphous and disordered materials such as glasses, \(n\) takes up the value as 2 because of the indirect transitions of rare earth ions. The band gap values are given in Table 1. The exponential tail seen at the absorption edge of the glasses indicates that there may be some sort of defect states or disorderness present which are created due to the incident high energy ultra-violet radiation and heavy element doping such as Dy, Eu. The exponential tail is otherwise called Urbach tail, and it determines the number of defects or disorderness present in the glasses. The defects are quantified in terms of Urbach energy (eV) which can be obtained by plotting In (\(\alpha\)) against \(h\nu\) (photon energy), and then taking the inverse of the slope value obtained [20] as shown for ZABSDE1 glass in Fig. 3. These values are presented in Table 1. The reverse trend in bandgap energy and Urbach energy values suggest that, the more the localized energy levels/defects in the glass system, the less the bandgap energy becomes. Thus, the glasses with higher bandgap values show lower Urbach energy values.
3.2 Photoluminescence measurements
3.2.1 Excitation and emission studies
The excitation spectra for the co-doped glasses were recorded at 575 and 613 nm wavelengths and are given in Fig. 4a, b, respectively. Under 575 nm, both Dy3+ singly doped glass and Dy3+/Eu3+ co-doped glasses showed Dy3+ peaks at 325 (4M17/2+6P3/2), 350 (6P7/2), 364 (4I11/2+6P5/2), 387 (4I13/2+4F7/2), 425 (4G11/2), 453 (4I15/2), and 473 nm (4F9/2) from 6H15/2 ground level [20]. But the Eu3+ ions do not promote any kind of transitions with 575 nm. Moreover, the intensity of the observed peaks decreases on co-doping with Eu3+ ions. At 2.5 mol% of Eu3+ dopant the Dy3+ peaks achieved a very low intensity as seen from Fig. 4a. With 613 nm wavelength, the Dy3+ singly doped glass shows very low intensity of Dy3+ peaks at 342, 350, 364, 387, 425, and 450 nm given in the inset of Fig. 4b. With addition of Eu3+ ions even the low intensity peaks of Dy3+ are suppressed completely and the only peak at 350 nm (6P7/2) is seen with minimum intensity in co-doped glasses. This means that 613 nm is an excitation source for Eu3+ ions which only triggers them to get excited to higher energy levels located at 319 (7F0→5H6), 362 (7F0→5D4), 382 (7F0→5G2), 393 (7F0→5L6), 414 (7F0→5D3), 465 (7F0→5D2), 525 (7F0→5D1) and 533 nm (7F1→5D1) [21].
The emission spectra recorded at λexc = 350 nm is shown in Fig. 5a. Under 350 nm, the Dy3+ singly doped glass features three emission bands from 4F9/2 excited level to ground levels lying at 482 (6H15/2), 575 (6H13/2) and 663 nm (6H11/2); whereas the Dy3+-Eu3+ co-doped glasses show five emission bands in which Eu3+ are located at 613 (5D0→7F2) and 701 nm (5D0→7F4) along with emissions of Dy3+ at 482 nm, 575 and 663 nm. On exciting Dy3+ ions the emission bands corresponding to Eu3+ are also observed in the spectra. Most importantly, increasing the Eu3+ concentration leads to the decrease in Dy3+ emission intensity and this assures the possibility energy transfer from Dy3+ to Eu3+. On exciting the glasses under 393 nm wavelength (Fig. 5b), the emission spectra show a steady decrease in the Dy3+ band at 482 nm whereas the Dy3+ peak at 575 nm splits up into two peaks i.e., the original peak at 575 nm (Dy3+) and newly formed peak at 590 nm (Eu3+). This spectral energy level splitting is seen when increasing the concentration of Eu3+ ions beyond 0.5 mol% (given in inset of Fig. 5b). The intense emission peak of Eu3+ seen at 613 nm (5D0 → 7F2) reaches a maximum height for ZABSDE3 glass i.e., at 1.0 mol% of Eu3+ co-doping. Above this concentration, the emission intensity decreased slowly. Hence, the concentration quenching was achieved for Eu3+ co-doping beyond 1.0 mol% under 393 nm excitation [16, 17]. This sort of concentration quenching in the co-doped glasses with the excitation source of the activator could be due to the energy transfer between activator ions (i.e., Eu3+ ions), possibly due to radiative re-absorption. Thus, ZABSDE3 glass is regarded as the optimum candidate with excitation in the near ultra-violet region (393 nm).
3.2.2 Spectral overlap and energy level diagram
Figure 6a depicts the overlap diagram that includes Dy3+ emission and Eu3+ excitation in singly doped glass. The PL (Photoluminescence spectroscopy) characteristics assure the blue and yellow emissions (Dy3+) as well as deep red emissions (Eu3+) from the glasses. The spectral overlap image reveals that the emission band of Dy3+ at 482 nm (4F9/2→6H15/2) exhibits an overlap with the excitation band of Eu3+ at 464 nm (7F0→5D2). This spectral overlap of donor ion’s emission spectrum (Dy3+) and acceptor ion’s excitation spectrum (Eu3+) indicates a migration occurring between them. Figure 6b shows the energy level diagram for Dy3+ and Eu3+ transitions. As seen from the excitation spectra, the two emission wavelengths at 575 and 613 nm are used for promoting the Dy3+ and Eu3+ ions to their upper excited levels. Later both the Dy3+ and Eu3+ ions reach one of their intermediate levels at 4F9/2 and 5D0 through non-radiative relaxation. On reaching the intermediate level at 4F9/2, Dy3+ ions transfer part of the energy to Eu3+ ions lying in the metastable state at 5D0. This is possible because the energy level of Dy3+ (4F9/2) is approximately 21,000 cm−1 which is slightly greater than the energy levels of Eu3+ at 5D1 (19,020 cm−1) and 5D0 (~ 17,277 cm−1) making the Dy3+ ions a resourceful sensitizer for Eu3+ ions [16]. The green arrow shown in Fig. 6b denotes the transfer of energy from Dy3+ to Eu3+. After the energy transfer process, Dy3+ ions reach their ground levels at 6H15/2, 6H13/2 and 6H11/2 by giving visible emissions in blue (482 nm), yellow (575 nm) and red (663 nm) regions, respectively. Similarly, the Eu3+ ions absorb the energy from Dy3+ and undergo emissions to the lower levels at 7F1 (590 nm), 7F2 (613 nm), 7F3 (652 nm), 7F4 (701 nm).
3.2.3 Decay analysis of Dy 3+ -Eu 3+ co-doped glasses
The decay profile of the 4F9/2 → 6H15/2 transition of Dy3+ ions under 350 nm excitation and 575 nm emission is given in Fig. 7a. All the glasses show a bi-exponential behaviour under 350 nm. The lifetime values are drawn out using ExpDec2 Fit as shown in Fig. 7b. The bi-exponential fitting equation is given as [20].
where \({A}_{1}\) and \({A}_{2}\) are the constants, \({t}_{1}\) and \({t}_{2}\) are the luminescence decay times. Using the two lifetime values, the average lifetime is determined via the Eq.
The calculated lifetime decreases with increasing Eu3+ concentrations, and the values are obtained at 512.34 µs, 497.53 µs, 481.64 µs, 470.94 µs, 462.18 µs, 445.53 µs and 433.74 µs corresponding to samples ZABSDE0, ZABSDE1, ZABSDE2, ZABSDE3, ZABSDE4, ZABSDE5, ZABSDE6, respectively. This decrease in lifetime of Dy3+ ions in level 4F9/2 clearly indicates that energy transfer occurs from Dy3+ ions to Eu3+ ions [22]. Similarly, the decay study of the 5D0 → 7F2 transition of Eu3+ ions under 350 nm excitation and 613 nm emission is given in Fig. 8a. The representative fitting plot is given in Fig. 8b. The bi-exponential behaviour is observed in this case also. Here the excitation of Dy3+ ions improved the lifetime of 5D0 state of Eu3+ ions though energy transfer. The lifetime of the 5D0 level of Eu3+ ions record a highest value for 1.0 mol% (ZABSDE3) glass; then the lifetime values are decreased beyond this concentration. The decrease in lifetime is due to the more amount of energy transfer from Dy3+ to Eu3+ ions possibly beyond 1.0 mol%. The values are given in Table 2. The lifetime values are then used to calculate other parameters such as energy transfer efficiency (\({\eta }_{ET}\)), and the probability of energy transfer (\({P}_{ET }\)) applying the following formulas [23].
where \({\tau }_{{d}_{o}}\) and \({\tau }_{d}\) are the inherent decay times of donor (Dy) in the presence and absence of acceptor (Eu). The obtained values are listed in Table 2. The increase in energy transfer efficiency is seen from 2 to 15% with increasing the Eu3+ ions.
3.2.4 Inokuti-hirayama fitting
The luminescence quenching via non-radiative energy transfer from 4F9/2 (Dy3+) level to 5D0 (Eu3+) level can be explained by Inokuti-Hirayama (I-H) model. Using the I-H model, it is simpler to identify the nature of energy transfer between the donor and the acceptor. The non-exponential decay curves are fitted to the I-H model which implies the following relation,
Here, S represents the interaction type such that, \(S\) = 6, 8, 10 corresponds to dipole-dipole (d-d), dipole-quadrapole (d-q), and quadrapole-quadrapole (q-q) interactions, respectively. The I-H fitting plot for ZABSDE3 glass is shown in Fig. 9. The best linear fit is seen for \(S\) = 6, with the R2 values obtained at 0.99. From the fitting table (inset of Fig. 9) the term Q stands for the energy transfer parameter given as.
where C is the concentration of acceptor ions (Eu3+), \({R}_{O}\) is the critical energy transfer distance or the distance of a donor-acceptor pair, \(\varGamma \left(1-\frac{3}{S}\right)\) is a constant value which equals to 1.77 for dipole-dipole (\(S=6\)), 1.43 for dipole-quadrapole (\(S=8\)), and 1.30 for quadrapole-quadrapole (\(S=10\)) [22]. From Eq. (7) the \({R}_{O}\) value is obtained. Using the \({R}_{O}\)value the donor-acceptor interaction parameter is calculated as follows,
The calculated values are grouped in Table 2. The energy transfer (Q), energy transfer efficiency \(\left({\eta }_{ET}\right)\), and probability of energy transfer (\({P}_{ET})\) are all found to increases with increasing Eu3+ concentration, while the critical energy transfer distance (\({R}_{O}\)), and donor-acceptor interaction parameter (\({C}_{DA}\)) decreases with increasing Eu3+ concentration. Thus, from the I-H fitting, it can be deducted that the type of energy migration between Dy3+ and Eu3+ is ‘dipole-dipole’ type when the condition \(S=6\) is satisfied.
3.2.5 Dexter energy transfer model
The Dexter energy transfer model is simple, and it is adopted when there is a spectral overlap between energy levels of donor and acceptor. The Dexter energy transfer is associated with the term ‘quenching’ such that the emission spectra is wholly considered. The Dexter’s energy transfer (ET) formula along with Reisfeld’s approximation is used to determine the type of energy migration in Dy3+ → Eu3+ using the following relation [23, 24].
where \({\eta }_{o}\) and \(\eta\) represent the quantum efficiency of the Dy3+ in absence and presence of Eu3+, respectively; C denotes the concentration of sensitizer (Dy) and activator (Eu) in mol% and \(n\) stands for the type of interaction i.e., \(n\) = 6, 8, 10 for dipole-dipole, dipole-quadrapole, and quadrapole-quadrupole. Equation (9) can be related to luminescence intensities given as
Here, \({I}_{SO}\) and \({I}_{S}\) denote the luminescence intensity of Dy3+ without Eu3+ and with Eu3+, respectively when the glasses are excited at 350 nm. By plotting \(\frac{{I}_{SO}}{{I}_{S}}\) versus \({C}^{n/3}\), the best linear fit can be determined when \(n\) = 6, 8, 10. From Fig. 10, it is seen that the best linear fit is obtained best for \(n\) = 6, with R2 = 0.9874 suggesting the dipole-dipole type of interaction between Dy3+ and Eu3+. The latter finding is in accordance with the I-H fitting method.
3.2.6 Color coordinates and correlated color temperatures
The color estimation for the glasses under different excitations were provided by CIE-1931 chromaticity diagram. The CIE plots of glasses under 350 and 393 nm excitations are given in Fig. 11a and Fig. 11b, respectively, and their color coordinates (x, y) are listed in Table 3. Under 350 nm, the CIE chromaticity coordinates are found to move from a neutral white light (0.370, 0.401) to warm white light (0.427, 0.376) with increased Eu3+ content. This change is due to the energy transfer from Dy3+ to Eu3+. Moreover, the excitation under 393 nm shifts the coordinates from cool white light to reddish region (0.360, 1560). To know about the color tuneability behaviour of the glasses under different excitation sources, ZABSDE3 glass was selected and excited at different wavelengths at 350 nm, 364 nm, 382 nm, and 393 nm. Under all these excitations, it is noted from Fig. 11c that the color emission from the glass moves from white light region to reddish region. The correlated color temperature (CCT) values were evaluated from the equation given as [25, 26].
The variation in CCT values is seen with varying the Eu3+ concentration (Table 3). Thus, in the present work, addition of Eu3+ ions played a significant role in color emission from neutral white light to warm white light. Therefore, the glasses can be suitable for color tuneable LEDs and near ultra-violet W-LEDs application.
4 Conclusion
Dy3+-Eu3+ co-doped glasses were prepared using a high-temperature melt-quenching method. The UV-Visible-NIR study revealed the presence of Dy3+ and Eu3+ transitions with overlap of Dy-Eu peaks. The bandgap and Urbach energy values followed reverse trend with varying Eu3+ ions. Emission studies revealed that Dy3+ ions exhibit energy transfer to Eu3+ ions through non-radiative process under 350 nm excitation. The dipole-dipole type of interaction between Dy3+ and Eu3+ is determined using the I-H fitting model and dexter energy model. The chromaticity coordinates obtained for lower concentration of Eu3+ is found to be consistent for white light emission compared to higher Eu3+ concentration. The co-doped glass showed color tuneability behaviour with different excitations in the near UV to visible region, favorable for near-ultraviolet light emitting diode applications.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
S. Dutta, S. Som, S.K. Sharma, Luminescence and photometric characterization of K+ compensated CaMoO4:Dy3+ nanophosphors. Dalton Trans. 42, 9654–9661 (2013). https://doi.org/10.1039/C3DT50780G
S. Xiaojiao Kang, H. Lu, D. Wang, W. Ling, Lu, Tricolor- and White Light-Emitting Ce3+/Tb3+- coactivated Li2Ca4Si4O13 phosphor via energy transfer. ACS Omega 3(12), 16714–16720 (2018). https://doi.org/10.1021/acsomega.8b01952
S. Som, P. Mitra, V. Kumar, V. Kumar, J.J. Terblans, H.C. Swart, S.K. Sharma, The energy transfer phenomena and colour tunability in Y2O2S:Eu3+/Dy3+ micro-fibers for white emission in solid state lighting applications. Dalton Trans. 43, 9860–9871 (2014). https://doi.org/10.1039/C4DT00349G
Y. Sun, F.Y.M. Liao, J. Ma, X. Wang, D. He, W. Gao, J. Knight, Hu. Lili, Visible emission and energy transfer in Tb3+/Dy3+ co-doped phosphate glasses. J. Am. Ceram. Soc. 103(12), 6847–6859 (2020). https://doi.org/10.1111/jace.17391
V. Rajeswara Rao, L. Lakshmi Devi, C.K. Jayasankar, W. Pecharapa, J. Keawkhao, Shobha Rani Depuru, luminescence and energy transfer studies of Ce3+/Dy3+ doped fluorophosphate glasses. J. Lumin. 208, 89–98 (2019). https://doi.org/10.1016/j.jlumin.2018.11.053
Y. Yimeng, H. Huo, H. Zhang, T. Zhao, Q. Wang, X. Zou, C. Su, Preparation and luminescence of Dy3+ doped glass-ceramics containing ZnMoO4. J. Non Cryst. Solids 569, 120990 (2021). https://doi.org/10.1016/j.jnoncrysol.2021.120990
B. Fan Liao, W. Shen, Y. Wu, J. Zhang, Hu, A study on the anti-thermal Dy3+/Eu3+ co-doped BaLa4Si3O13 Red Phosphors for White-Light -Emitting Diodes and Optical Thermometry Applications. Ind. Eng. Chem. Res. 60, 2931–2943 (2021). https://doi.org/10.1021/acs.iecr.0c05996
H. Jialiang Niu, W. Ding, Z. Zhou, D. Zhang, X. Bai, Wang, Investigations on energy transfer mechanism and tunable luminescent properties of co-doped Ca9La(PO4)7:Dy3+,Eu3+ phosphors. Appl. Phys. A 126, 378 (2020). https://doi.org/10.1007/s00339-020-03563-w
K. Anilkumar, S. Damodaraiah, S. Babu, V. Reddy Prasad, Y.C. Ratnakaram, Emission spectra and energy transfer studies in Dy3+ and Dy3+/Eu3+ co-doped potassium fluorophosphate glasses for white light applications. J. Lumin. 205, 190–196 (2019). https://doi.org/10.1016/j.jlumin.2018.09.007
E. Shelby James, Introduction to Glass Science and Technology (Royal Society of Chemistry, London, 2015)
D. Ruiwang Liu, M. Wang, L. Chen, Y. Liu, F. Zhou, Zeng, Zhongmin Su, luminescence, energy transfer properties of Dy3+/Eu3+ coactivated neutral and warm white emissions GSBG glasses. J. Lumin. 237, 118180 (2021). https://doi.org/10.1016/j.jlumin.2021.118180
A.K. Kaushal Jha, M. Vishwakarma, D. Jayasimhadri, K. Haranath, Jang, Multicolor emission and energy transfer dynamics in thermally stable Dy3+/Eu3+ co-doped ZPBT glasses for epoxy free w-LEDs application. J. Non Cryst. Solids 553, 120516 (2021). https://doi.org/10.1016/j.jnoncrysol.2020.120516
D.V. Krishna Reddy, T. Sambasiva Rao, S. Taherunnisa, A. Suchocki, Y. Zhydachevskyy, M. Piasecki, M. Rami, Reddy, Tunable white light by varying excitations in yttrium alumino bismuth borosilicate glasses co-doped with Dy3+- Eu3+ for cool WLED applications. J. Non Cryst. Solids 513, 167–182 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.03.011
G. Wenli Zhou, X. Wang, L. Zheng, J. Yu, Z. Zhang, S. Qiu, Lian, Tunable colors and applications of Dy3+/Eu3+ co-doped CaO-B2O3-SiO2 glasses. J. Am. Ceram. Soc. 102(10), 5890–5898 (2019). https://doi.org/10.1111/jace.16442
Z. Jingxi An, Y. Zhang, ZhiYuan Qiu, Y. Fu, F. Zhou, Zeng, Luminescence properties of borosilicate glass doped with Ce3+/Dy3+/Eu3+ under ultraviolet excitation for white LED. J. Non Cryst. Solids 503–504, 208–213 (2019). https://doi.org/10.1016/j.jnoncrysol.2018.09.050
M.K. Nisha Deopa, P.R. Sahu, R. Rani, A.S. Punia, Rao, Realization of warm white light and energy transfer of Dy3+/Eu3+ co-doped Li2O-PbO-Al2O3-B2O3 glasses for lighting applications. J. Lumin. 222, 117166 (2020). https://doi.org/10.1016/j.jlumin.2020.117166
P. Yu, W. Guo, R. Zhang, L. Su, J. Xu, White and tunable light emission in Eu3+, Dy3+ codoped phosphate glass. Opt. Mater. 114, 110939 (2021). https://doi.org/10.1016/j.optmat.2021.110939
J. Tauc, Optical properties and electronic structures of amorphous Ge and Si. Mater. Res. Bullet 3(1), 37–46 (1968). https://doi.org/10.1016/0025-5408(68)90023-8
E.A. Davis, N.F. Mott, Conduction in non-crystalline systems V, Conductivity, optical absorption, and photoconductivity in amorphous semiconductors. Taylor& Francis 22(179), 0903–0922 (1970). https://doi.org/10.1080/14786437008221061
M. Monisha, N. Mazumder, S.K. Melanthota, B. Padasale, H. Aljawhara, M.I. Almuqrin, N. Sayyed, D. Karunakara, Sudha, Kamath, Enhancement of dysprosium oxide doped zinc alumino borosilicate glasses in thermal, optical and luminescence domain for solid state lighting application. Opt. Mater. 128, 112447 (2022). https://doi.org/10.1016/j.optmat.2022.112447
A. Vinod Hegde, H. Wagh, C.S. Hegde, S.D. Dwaraka Vishwanath, Kamath, Spectroscopic investigation on europium doped heavy metal borate glasses for red luminescent applications. Appl. Phys. A 123, 302 (2017). https://doi.org/10.1007/s00339-017-0914-5
M. Vijayakumar, K. Marimuthu, Tailoring the luminescence of Eu3+ co-doped Dy3+ incorporated aluminofluoro-borophosphate glasses for white light applications. J. Lumin. 178, 414–424 (2016). https://doi.org/10.1016/j.jlumin.2016.06.016
F. Bin, W. Zhao, L. Han, Eu3+ co-doped Sr3Gd(PO4)3:Dy3+ phosphors: luminescence properties and color-tunable white-light emission for NUV-WLEDs. Appl. Phys. A 126, 260 (2020). https://doi.org/10.1007/s00339-020-3444-5
G. Li, D. Geng, M. Shang, C. Peng, Z. Cheng, J. Lin, Tunable luminescence of Ce3+/Mn2+ -coactivated Ca2Gd8(SiO4)6O2 through energy transfer and modulation of excitation: potential single-phase white/yellow-emitting phosphors. J. Mater. Chem. 21, 13334 (2011). https://doi.org/10.1039/C1JM11650A
C.-H. Huang, T.-W. Kuo, T.-M. Chen, Novel red-emitting Phosphor Ca9Y(PO4)7: Ce3+, Mn2+ with energy transfer for fluorescent lamp application. ACS Appl. Mater. Inter. 2(5), 1395–2010 (2010). https://doi.org/10.1021/am100043q
C.S. McCamy, Correlated color temperature as an explicit function of chromaticity coordinates. Color. Res. Appl. 17, 142–144 (1992). https://doi.org/10.1002/col.5080170211
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MM - Writing Original draft; MIS - Validation, Conceptualization; NM - Lifetime measurements; JA - Writing, Editing and Proof correction; SDK - Writing, Editing & Proof Reading.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monisha, M., Sayyed, M.I., Mazumder, N. et al. Color tuneability behaviour and energy transfer analysis on Dy3+-Eu3+ co-doped glasses for NUV-WLEDs application. J Mater Sci: Mater Electron 34, 487 (2023). https://doi.org/10.1007/s10854-022-09602-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09602-x