Abstract
Avalanche phenomenon uses critical pump power to produce extreme nonlinear behavior from small disturbances, and has gradually become known. Here, it is reported that the strong green up-conversion emission produced in NaBi(WO4)2 phosphor by the positive feedback enhancement of the energy transfer process. The power dependence indicates that the photon avalanche process has occurred. Contrary to other up-conversion mechanisms, photon avalanche (PA) is a bifurcation phenomenon: avalanches occur above the critical excitation power. The experimental results are analyzed using the rate equation model. The high-response photon avalanche process produced by Yb and Ho ions is discussed in detail. The results show that PA can not only improve the brightness and efficiency of up-conversion, but also has a wider range of applications than traditional up-conversion materials, especially in the detection material of temperature sensor plays an important role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, rare earth (RE)-doped luminescent materials have attracted more and more attention. The electric dipole transition and the magnetic dipole transition cause the photoluminescence of RE ions. In addition, one of the most valuable properties of these materials is “up-conversion (UC),” which converts low-energy photons (infrared or visible light) into high-energy photons through the process of multiphoton absorption or energy transfer, this makes UC phosphors widely used in optical temperature sensors and photoelectric sensor. Traditional sensors rely on the expansion principle of liquids and metals, or have low infrared conversion efficiency. Therefore, they cannot be used in harsh environments, and the sensing accuracy is low, which makes them no longer meet the needs of current social sciences, the UC fluorescence intensity ratio (FIR) technique is considered to be one of the state-of-the-art [1,2,3,4,5,6,7,8,9]. UC luminescence occurs through three mechanisms: (1) ground state absorption (GSA); (2) excited state absorption (ESA) and continuous energy transfer (ETU) from donor to up-conversion acceptor; (3) and photon avalanche. Among them, the PA process is relatively rare and is one of the most effective UC processes. In the PA process, when the pump power reaches the threshold, the number of excited particles rises sharply due to the cross-relaxation, resulting in strong up-conversion emission, due to these special characteristics, these materials have huge applications in various fields such as optical temperature measurement, laser devices, display devices, optical amplifiers, fingerprint printing, and biological imaging [10,11,12,13,14,15,16,17,18,19,20,21].
The biggest advantage of the PA process is that it not only has an extreme nonlinear effect but also a high efficiency, and there is no periodic photon emission or interference effect. The PA phenomenon was discovered for the first time in Pr3+ ion-doped crystals. It was found that when the excitation exceeds the critical pump laser intensity, the up-conversion luminescence suddenly increases. This special property quickly promoted development of other PA materials, such as high-efficiency up-conversion lasers. The PA phenomenon is mainly observed at low temperatures, with some exceptions at room temperature. Lahoz reported a very strong green band at room temperature in Ho3+-doped fluoroindium glass under excitation at 747 nm. Temporal dynamics based on strong excited state absorption and processes suggest that a photon avalanche mechanism occurs. Auzel F reported the photonic avalanche UC process and mechanism of erbium-doped ZBLAN glass at room temperature [22,23,24]. But its unique properties are not only limited to the application of luminescence, but also aroused interest in different fields. For example, a recent study found that through continuous wave pumping, the emission intensity of the 3F4 intermediate state energy level of Tm3+ ions non-linearly expands to the 26th power with the pumping intensity, and the application of super-resolution imaging has been realized. This also provides research ideas for the detection of the COVID-19 virus that has recently swept the world [1]. In the PA material doped with lanthanides, a single GSA triggers a chain reaction between the ESA and Ln3+ ions in the cross-relaxation process, which will result in a large number of photon emission [1].
The crystal field around the dopant ion has a great influence on the UC efficiency. Compared with high symmetry crystals, low symmetry crystals will generate more asymmetric crystal fields around the dopant ions, thereby greatly improving the UC efficiency. For example, the NaBi(WO4)2(NBW) crystal, which has been studied more in recent years, has a non-centrosymmetric tetragonal crystal structure space group\(\overline{{\text{I}}} {\text{41}}/{\text{a}}\) I̅41/a [24], therefore, a more asymmetric crystal field will be generated around the rare earth ions, which increases the probability of the 4f-4f transition of the rare earth ions, and the NBW crystal has a disordered structure, which is due to the irregular distribution of Bi3+ and Na+ ions in the crystal lattice, which leads to an increase in emission intensity [25,26,27]. However, in order to achieve the expected photon avalanche phosphor, it is very important to select the appropriate RE ions. Since Ho3+ ions have intermediate sub-energy levels and stable 5I5 and 5I6 energy levels, these characteristics greatly promote the efficiency of excited state absorption and cross-relaxation processes [28].
Therefore, in this article, we report Yb3+ and Ho3+ co-doped NBW phosphors, and found that the cross-relaxation and energy cycling process of Ho3+ ions produce extreme nonlinear PA phenomenon. By obtaining the average of the number of photons and the rise time of the green light emission level with power, the photon absorption process was observed and verified, and the PA process was explained by the cycle mechanism.
2 Materials and characterization
A series of Yb3+/Ho3+ co-doped NaBi(WO4)2 phosphors were synthesized by hydrothermal method. High-purity sodium tungstate (Na2WO4·2H2O) (99.5%), holmium Oxide (Ho2O3) (99.99%), ytterbium oxide (Yb2O3) (99.99%), bismuth nitrate (Bi(NO3)3·5H2O) (99.9%) are used as starting materials. Before the reaction starts, the rare earth oxide is first dissolved in concentrated nitric acid to form nitrate (RE(NO3)3), and a certain amount of Na2WO4·2H2O is uniformly dissolved in 20 mL of deionized water. The amount of each chemical calculated according to the ingredients, nitrates RE(NO3)3, and Bi(NO3)3·5H2O are dissolved in 20mL of deionized water. Then slowly add the sodium tungstate solution to the nitric acid solution to form a suspension, and keep the mixed solution under acidic conditions and stir for 30 min, transfer it to a stainless steel autoclave (70 mL). In the experiment, the reaction kettle was kept at 220 ℃ for 18 h. After standing, it was naturally cooled to room temperature, and the reaction product was centrifuged. washed with deionized water and absolute ethanol 3 times, and then dried in air at 60 ℃ for 20 h to obtain the final sample.
Use CuK-α radiation source (k = 0.1546 nm, 40 kV, 20 mA) X-ray powder diffractometer (XRD-7000, SHIMADZU) in the range of 10°–80°, 5°/min scan rate to characterize the test sample. Scanning electron microscopy (SEM, EVOMA-25, ZEISS) was used for morphological studies. The absorption spectrum was measured by Perkin Elmer Co., Ltd. (Lambda 950 UV-VIS). A spectrophotometer (FLS920, Edinburgh Instruments) was used to measure the up-conversion emission spectra and fluorescence decay curves of the red and green emission under 980 nm excitation.
3 Results and discussion
3.1 Yb3+, Ho3+: NaBi(WO4)2 structure and morphology analysis
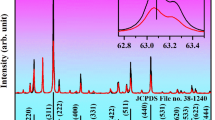
The XRD of the NBW phosphor doped with different concentrations of Yb3+ ions are depicted in Fig. 1a. All diffraction peaks are consistent with the diffraction peaks of standard card (JCPDS#52-1718), and no miscellaneous peaks are detected, indicating that the NBW: 1%Ho3+, x%Yb3+ compound has been successfully prepared, and the substitution of Yb3+, Ho3+ ions have no significant effect on the crystal structure. In addition, as shown in Fig. 1b, as the Yb3+ ion concentration increases, the diffraction peaks regularly shift to large angles. This is because Bi3+ has a larger radius and is larger than rare earth ions (Ho3+: 0.0901 nm, Yb3+: 0.0868 nm, Bi3+:0.096 nm). According to the Bragg equation \(2d\sin\theta =n\lambda\), when Bi3+ ions are replaced by Yb3+ and Ho3+ ions with a smaller ion radius, the lattice constant and the distance between planes (d) decrease, the diffraction angle increases. As a result, the main diffraction peak is shifted to large angle.
Figure 2a is the absorption spectrum of NBW phosphors, there are absorption peaks located at 451, 488, 528, and 655 nm, which are attributed to the 5I8 to 5G6, 5F2+5F3, 5F4+5S2, and 5F5 energy of Ho3+ ions, respectively [29, 30]. The optical band gap of NBW: 1%Ho3+, x%Yb3+ phosphor was calculated by Wood and Tauc formula [31], as shown below:
where Eg is the optical band gap, hυ is the incident photon energy, and B is the band adjustment constant. For transitions that are directly allowed, the value of the constant “m” is taken as 1/2. Figure 2b shows the curve drawn between (αhυ)2 and hυ for Ho3+ and Yb3+ co-doped NBW phosphors, with a direct optical band gap of 2.97 eV. As shown in Fig. 2c, d the optical band gap of NBW gradually decreases with the doping of rare earth ions. As we all know, one of the main factors affecting the luminous intensity is the microscopic morphology of the sample. Regular and uniform size is the most ideal microscopic morphology, and the sample exhibits high cumulative density, which can reduce light scattering in addition to obtaining high luminous efficiency. SEM images of representative samples NBW: 1%Ho3+, 5%Yb3+ are shown in Fig. 3a, b. Obviously, the samples observed under SEM are in sheet-like and multi-faceted morphology, which indicates that the crystallinity of the particles is very high. The element composition and uniformity of NBW: Yb, Ho were determined by EDS. The average atomic ratio Na/Bi/W is 0.06: 2.57: 15.52, which is close to the formula NaBi(WO4)2, and the element mapping image Fig. 3c–j also indicates the uniform distribution of elements in the phosphor particles.
3.2 Research on up-conversion of Yb, Ho: NaBi(WO4)2 phosphor
Figure 4 shows the UC emission spectrum and R/G emission intensity ratio of NBW: 1%Ho3+, x%Yb3+ measured at room temperature. The 5S2/5F4→5I8 of Ho3+ ion produces green emission at 541 nm, while the 5S2/5F4→5I8 transition produces red emission at 644 nm [32, 33]. Both red and green emission gradually increase with the increase of Yb3+ ion concentration (3 mol%→11 mol%) and the luminous intensity reaches the peak at 11 mol% Yb3+, and then there is concentration quenching, resulting in the luminous intensity at 13 mol% Yb3+ reduce. In order to understand the enhancement of red and green emission in depth, The energy level diagram in Fig. 5 shows the energy transfer process of Yb3+→ Ho3+ ions.
The energy transfer mechanism between Yb3+ and Ho3+ ions is described in detail in Fig. 5. In this work, Yb3+ ion is selected as the sensitizer, so we only consider the two energy levels of Yb3+, so its absorption cross section is very large. When Yb3+ ion absorbs 980 nm laser, it is excited from 2F7/2 state to 2F5/2 state, it can effectively transfer energy to Ho3+ ions. This makes it easy for the large amount of energy absorbed by Yb3+ ions to be transferred to Ho3+ ions [34]. In low concentration Yb3+ ion doping, the pump energy absorbed by Yb3+ ion passes ET1[2F5/2(Yb3+)+5I8(Ho3+)→2F7/2(Yb3+)+5I6(Ho3+), ET2[2F5/2(Yb3+)+5I6(Ho3+)→2F7/2(Yb3+)+5S2/5F4(Ho3+)], ET3[2F5/2(Yb3+)+5I7(Ho3+)→2F7/2(Yb3+)+5F5(Ho3+)] can fill the 5S2/5F4 and 5F5 energy levels, respectively, producing green and red emission [35, 36]. According to the analysis of Yb and Ho energy transfer in the figure above, the UC emission at 541 and 644 nm occurs through a two-photon process. However, due to the PA process, our results show that the average number of photons emitted by UC is greater than 5.
3.2.1 Avalanche mechanism
According to reports, the conventional PA mechanism has two notable features: The first typical feature is the non-linear increase of the slope n of UC emission under the pump power threshold. This can be explained by the “s”-shaped power dependence. The increase is usually caused by positive feedback filling the intermediate energy level [37, 38]. The observation in Fig. 6 is similar to this situation. The second is to use square wave excitation technology to perform instantaneous pacing measurement, where the conventional PA mechanism shows a sudden non-linear upward trend at the pump power threshold, while in continuous two-photon absorption only a linear upward trend is displayed.
In this effect, pumping corresponds to the absorption of intermediate excited state energy levels (metastable state), rather than the common pure electron absorption from ground state to excited state. Generally speaking, this method of pumping is impossible, but the intermediate energy level starts to be filled through the PA process, and then the pump light is strongly absorbed. Two necessary conditions must be met to construct this process: (1) Excited state absorption (ESA) is produced by a long-lived intermediate energy level [39]; (2) The intermediate energy level is filled by some effective cross-relaxation processes [40]. Therefore, this puts special requirements on the doped ions and concentration, and the host material. In addition to the disparity in the pumping scheme, there are also differences in other aspects between the ordinary up-conversion and the PA process. Generally speaking, the non-linear dependence and threshold value of the PA process on the pump power have a much longer stabilization time than the normal UC luminescence process, and the stabilization time is related to the pump intensity.
In Fig. 6, we find the nonlinear behavior between the UC emission intensity and laser power, and this mutation phenomenon is only found in the visible light emission band. This means that not only the excited state absorption, but also the existence of a cyclic process, instead of the traditional PA process. Recently, Shivkumar et al. proposed the Hetrolooping Enhanced Energy Transfer (H-LEET) process to explain this process. H-LEET process energy transfer up-conversion and positive feedback/cyclic process and non-zero ground state absorption. The biggest difference between the PA process and the H-LEET process is the non-zero ground state absorption. But the nonlinear power dependence of the H-LEET and PA processes is similar [37, 41]. Goldner et al. discussed the theoretical limitations of the PA process and proposed a cross-relaxation enhanced excited state absorption (CRESA) mechanism [42]. However, CRESA is based on the inherently less efficient GSA/ESA process. Only described in terms of single crystal.
In order to describe the energy transfer method in the PA process in more detail, we have omitted other irrelevant energy levels, so the simplified model of the cyclic process is shown in Fig. 7. We use the following up-conversion and cycling process to explain the PA process found in NBW. First, 980 nm photons fill the 5I6 energy level through GSA. Through three different processes, 5I6 energy level ions are further promoted to 5S2:5F4 energy level; through energy transfer processes, CR and ESA energy transfer processes (Fig. 7). In Ho3+, a few energy transitions are very similar, namely 5I8→5I7 and 5S2→5I4 (resonance), 5I4→5I6 and 5I7→5I6 (non-resonance), 5S2→5I6 and 5I8→5I6 (resonance), these transfer processes make cross. The relaxation process fills the 5S2 energy level, resulting in an avalanche effect [9].
In the presence of Yb3+ ions, energy passes forward energy transfer (FT) [Ho3+→Yb3+;(5S2:5F4, 2F7/2)→(5I6, 2F5/2)] and reverse energy transfer (BT) [Yb3+→Ho3+; (2F5/2,5I8)→(2F7/2,5I6)] proceed [9], Therefore, the energy transfer and CR process compete with each other, forming two cycles, namely Ho3+-Ho3+ cycles caused by cross-relaxation between ions and FT/BT cycles (Fig. 7). The excited state at the 5I6 energy level. The two Ho3+ ions are produced by the Ho3+ ions at the 5S2:5F4 energy level from two cycles, and they can also be used to absorb incident pump light. This is a process of multiplication, and four excited Ho3+ ions are generated during the next cycle. Therefore, the PA process took place.
In this work, the PA process is based on the CR, FT, and BT processes that lead to an increase in the number of photons in the 5I6 and 5S2:5F4 energy levels [35], therefore, we use the theoretical rate equation model to evolve the total energy and its changes over time [43]:
For Ho3+ ion:
For Yb3+ion:
Among them, RESA and RGSA are the pumping rates of the excited state and ground state, respectively; Mi and Ni are the population in ith level of Yb3+ and Ho3+ ions; rFT is the forward transfer efficiency [(N2,M0)→(N1,M1)]; RCR is the CR rate of the entire process;, rBT is the reverse transfer efficiency [(M1, N0)→(M0, N1)], Bji is branch ratio of j→i; Ai, Ai0 are the radiative and non-radiative transition rates of the energy level [44, 45].
We calculated the cross-relaxation, forward and reverse energy transfer rates, and analyzed the cyclic process between Yb3+ and Ho3+ ions in depth (Table 1).
The non-radiative relaxation process dominates only when the ion concentration is high, so the life value calculated by the exponential function fitting has a large error. In this case, only the effective relaxation rate and average lifetime can be calculated. We calculated the effective lifetime of the 2F5/2 energy level of Yb3+ ion and the 5S2, 5F4 energy level of Ho3+ ion using the following relationship:
where I(t) represent the emission intensity at time t after the incident beam is completely cut off. As the concentration of Ho3+ increases, the lifetime of red and green emission decreases significantly due to the cross-relaxation process. Figure 8 shows the decay curves of 5S2 and 5F4 levels. The CR rate WCR can be calculated by the following relationship [46]:
where x is the concentration of Yb3+ ions. When the CR rate is from 3→5 mol%, there is 49 S− 1. When it increases to 11 mol%, there is 380 S− 1. Therefore, under the condition of ytterbium ion co-doping, the energy transfer process occurs between Ho3+→Yb3+→Ho3+ ions.
4 Conclusion
Under the excitation of 980 nm laser, the abnormal “s”-shaped pump power dependence of UC emission in NBW: Yb, Ho phosphor was observed. It was found that the 5S2/5F4→5I8 of Ho3+ ion produces green emission at 541 nm, while the 5S2/5F4→5I8 transition produces red emission at 644 nm. Both red and green emission gradually increase with the increase of Yb3+ ion concentration (3 mol%→11 mol%) and the luminous intensity reaches the peak at 11 mol% Yb3+, and then there is concentration quenching, and the cross-relaxation and energy cycling process of Ho3+ ions produced an extreme nonlinear photon avalanche. By obtaining the average of the number of photons and the rise time of the green light emission level with power, the photon absorption process was observed and verified. The cyclic mechanism explains the PA process, that is, the CR, FT, BT process causes the number of photons at the 5I6 and 5S2/5F4 energy levels to double. At the same time, the theoretical results analyzed by the rate equation model are consistent with the experimental results. These results have important application value in the detection material of temperature sensor plays an important role, it also provides unprecedented opportunities for technological innovation.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
C. Lee, E. Xu, Y.W. Liu, A. Teitelboim, K.Y. Yao, A. Fernandez-Bravo, A. Kotulska, S.H. Nam, Y.D. Suh, A. Bednarkiewicz, B.E. Cohen, E.M. Chan, PJ. Schuck. Nature. 589, 230–235 (2021)
S.K. Singh, K. Kumar, S.B. Rai, Sens. Actuat A-Phys 149, 16–20 (2009)
R. Dey, V.K. Rai, Dalton T. 43, 111–118 (2014)
F. Wang, Y. Han, C.S. Lim, Y.H. Lu, J. Wang, J. Xu, H.Y. Chen, C. Zhang, M.H. Hong, XG. Liu. Nature. 463, 1061–1065 (2010)
Y. Dwivedi, S.B. Rai, J. Am. Ceram. Soc. 93, 727–731 (2010)
E. Downing, L. Hesselink, J. Ralston, R. Macfarlane. Science. 273, 1185–1189 (1996)
P. Zhang, W. Steelant, M. Kumar, M. Scholfield, J. Am. Ceram. Soc. 129, 4526–4527 (2007)
AS. E.B. Gouveia-Neto, L.A. Costa, Bueno, SJL. Ribeiro. Journal Alloy Compd. 375, 224–228 (2004)
Y. Dwivedi, A. Bahadur, S.B. Rai, J. Appl. Phys. 110, 043103 (2011)
A. Kumar, A. Bahadur, J. Alloy Compd 857, 158196 (2020)
M.F. Joubert, Opt. Mater. 11, 181–203 (1999)
A. Bednarkiewicz, E. Chan, A. Kotulska, L. Marciniak, K. Prorok. Nanoscale Horiz. 4, 881–889 (2019)
M.A. Mahdia, S.R. Yousefi, L.S. Jasima, M. Salavati-Niasari, Int. J. Hydrogen Energ. 47, 14319–14330 (2022)
S.R. Yousefi, H.A. Alshamsi, O. Amiri, M. Salavati-Niasari, J. Mol. Liq 337, 116405 (2021)
S.R. Yousefi, D. Ghanbari, M. Salavati-Niasari, M. Hassanpour, J. Mater. Sci-Mater El 27, 1244–1253 (2015)
S.R. Yousefi, M. Ghanbari, O. Amiri, Z. Marzhoseyni, P. Mehdizadeh, M. Hajizadeh-Oghaz, M. Salavati-Niasari, J. Am. Ceram. Soc. 104, 2952–2965 (2021)
S.R. Yousefi, A. Sobhani, H.A. Alshamsic, M. Salavati-Niasari, RSC Adv. 11, 11500–11512 (2021)
S.R. Yousefi, O. Amiri, M. Salavati-Niasari, Ultrason. Sonochem 58, 104619 (2019)
S.R. Yousefi, M. Masjedi-Arani, M.S. Morassaei, M. Salavati-Niasari, Int. J. Hydrogen Energ. 44, 24005–24016 (2019)
M. Salavati-Niasari, A. Sobhani, S.R. Yousefi, J. Soc. Powder Technol. Japan 28, 1258–1262 (2017)
S.R. Yousefi, D. Ghanbari, Masoud Salavati Niasari. J Nanostruct 27(2), 1244–1253 (2017)
H. Deng, S.H. Yang, S. Xiao, H.M. Gong, Q.Q. Wang, J. Am. Ceram. Soc. 130, 2032–2040 (2008)
F. Lahoz, I.R. Martín, V.L. Guadalupe, J. Mendez-Ramos, V.D. Rodriguez, UR. Rodriguez-Mendoza. Opt Mater. 25, 209–213 (2004)
F. Auzel, Y.H. Chen, D. Meichenin, J. Lumin. 60, 692–694 (1994)
J.H. Xie, L.Q. Chen, H. Tang, X.F. Yu, C.B. Wang, X.Y. Mi, Q.S. Liu, XY. Zhang. J Lumin. 219, 116841 (2019)
M.H. Li, L.L. Wang, W.G. Ran, Z.H. Deng, C.Y. Ren, JS. Shi. Ceram Int. 43, 6751–6757 (2017)
C.L. Ma, H. Cui, F.F. Li, J. Wang, X.X. Wu, J. Zhang, Q. Zhou, J.H. Liu, QL. Cui. J Solid State Chem 200, 246–250 (2013)
T.Q. Zhang, W.L. Yang, H.B. Li, X.Y. Wang, X.L. Jiang, Z. Leng, W.L. Yang, C. Li, H. Lin, H.S. Liu, K.K. Huang, C. Li, F.M. Zeng, ZM. Su. J Lumin. 240, 118432 (2021)
A.M. Pires, MR. Davolos. Chem Mater. 13, 21–27 (2001)
T. Jiang, Y. Tian, M.M. Xing, Y. Fu, X.M. Yin, H. Wang. Mater Res Bull 98, 328–334 (2018)
X.L. Jiang, X.Y. Wang, X.M. Shi, H.Y. Sha, W.J. Yang, W.L. Yang, Z. Leng, H. Lin, Z.M. Su, C. Li, F.M. Zeng, Opt. Mater. 113, 110873 (2021)
X.C. Yu, Y.B. Qin, M.L. Gao, L. Duan, Z.Q. Jiang, L. Gou, P. Zhou, Z. Li, J. Lumin. 153, 1–4 (2014)
Z.F. Shan, D.Q. Chen, Y.L. Yu, P. Huang, F.Y. Weng, H. Lin, YS. Wang. Mater Res Bull. 45, 1017–1020 (2010)
M.M. Xing, W.H. Cao, H.Y. Zhong, Y.H. Zhang, X.X. Luo, F. Yao, W. Feng, T. Pang, X.F. Yang, J. Alloy Compd. 509, 5725–5730 (2011)
X.J. Tan, S.L. Xu, F.H. Liu, X.Y. Wang, B.A. Goodman, D.K. Xiong, W. Deng. J Lumin 209, 95–101 (2019)
J.F. Tang, C.H. Cheng, Y.J. Chen, YD. Huang. J Alloy Compd. 609, 268–273 (2014)
G.Y. Chen, H.J. Liang, H.C. Liu, G. Somesfalean, Z.G. Zhang, J. Appl. Phys. 105, 114315 (2009)
R. Scheps, Prog Quant. Electron. 20, 271–358 (1996)
A. Franois, Chem. Rev. 104, 139–173 (2004)
E.S. Levy, C.A. Tajon, T.S. Bischof, J. Iafrati, A. Fernandez-Bravo, DJ. Garfield. Acs Nano. 10, 8423–8433 (2016)
S. Sivakumar, V. Veggel, P. May, J. Am. Ceram. Soc. 129, 620–625 (2007)
P. Goldner, F. Pelle, Opt. Mater. 5, 239–249 (1996)
E. Osiac, I. Sokólska, S. Kück, Phy Rev. B 65, 235119 (2002)
E. Osiac, I. Sokólska, S. Kück. J Lumin. 94, 289–292 (2001)
X.F. Wang, S.G. Xiao, Y.Y. Bu, X.L. Yang, JW. Ding. Opt Lett. 33, 2653–2655 (2008)
A.I. Burshtein, J. Lumin. 34, 167–188 (1985)
Acknowledgments
This work was supported by Government Funded Projects (627010104), International Science and Technology Cooperation Project of Jilin Province Science, Technology Department (20200801038GH), The Open Project of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry (Jilin University) (No.2021-19). Thanks to Professor Ligong Zhang, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences for his help in testing.
Author information
Authors and Affiliations
Contributions
HQ: conceptualization, data curation, formal analysis, writing—original draft. TZ: data curation, software, formal analysis, writing—editing. XJ: formal analysis, writing—review. HW: formal analysis, methodology. WY: investigation, methodology. CL: Conceptualization, supervision, funding acquisition, resources, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qian, H., Zhang, T., Jiang, X. et al. Visible photon avalanche up-conversion of Yb3+ and Ho3+ doped NaBi(WO4)2 phosphors under excitation at 980 nm. J Mater Sci: Mater Electron 33, 22718–22727 (2022). https://doi.org/10.1007/s10854-022-08963-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08963-7