Abstract
The melting quenching technique is used to create lithium tetraborate glasses with the following composition (in mol %): 80 Li2B4O7-(20-x) Nd2O3-x Cu metal or x CuO mol% (x = 0, 5, 10, 15, and 20). X-ray diffraction (XRD), optical absorption, photoluminescence, thermal analysis, and Fourier transmission infrared (FTIR) were studied. The optical absorption spectra indicate the presence of Nd3+ and Cu2+ ions. The absorption spectra determine the Judd–Ofelt intensity parameters (Ωλ), the oscillator strengths, and bonding parameters. The high value of Ω2 indicates the increasing covalent bonding within the host structure. The ratio between emission intensities and excitation indicates that glass samples are used as laser sources. The photoluminescence spectra revealed neodymium ion emission. The glasses containing 20 mol % Nd2O3 (free from copper) emitted light at 1053 nm. The strength of emission peaks decreases as copper concentration increases according to the quenching effect. The findings indicate that the samples investigated are suitable for use in solid-state lasers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, there has been a practical and scientific movement to research glass samples containing rare earth for possible use in optical devices [1,2,3,4].
Applications of the optical device have grown to include a wide range of possibilities, leading scientists to examine glass doped with rare-earth ions.
One of the most often used technical materials is glass. It is crucial to look at how the glass structure interacts with the properties.
One of the glass formers is boron. It has three to four coordinations and covalent B–O bonds with high bond strength [5,6,7,8,9].
Lithium lead borate glass-containing copper was studied by Rao et al., Kashif, and Ratep [10, 11]. They found that copper ions have a dual nature and enter as either a glass former or a glass modifier. The optical properties of glass samples containing copper change according to the valence state of copper ions, such as (Cu+) or (Cu2+).
The Nd3+ ions, out of all the rare-earth ions, have strong NIR emission transition peaks at 900, 1053, and 1330 nm. Telecommunication applications benefit from emissions at 1053 and 1330 nm [12,13,14,15]. In the medical field, these lasers employ to treat tracheal tumors and anticoagulant therapy [15].
Materials doped with trivalent rare-earth Nd3+ ions are optical materials in the field of lasers and optical waveguides, owing to the well-known special features of Nd3+ ions [16,17,18].
Nageswara Raju [19] investigated the optical characteristics of cadmium bismuth borate glasses containing neodymium oxide. They discovered that Nd–O bonds have more covalency, and Nd3+ sites are more asymmetric.
Gupta et al. [20] studied the Nd2O3–Zinc–Boro-Bismuthate glass system. In general, the results indicate that samples with a high amount of Bi2O3 can be a good material for laser emission (infrared laser).
Pascuta et al. [21] studied the influence of copper metallic on the optical characteristics of phosphate glasses co-doped with samarium ions. They obtained that the UV–Vis spectra reveal the presence of Cu2+ and Sm3+ ions in glass samples.
The presence of samarium ions causes the emission, which diminishes with an increasing copper concentration in a phenomenon known as the quenching effect.
Kashif et al. [22] investigated the substituting copper or copper oxide for lithium tetraborate on the physical properties of glass samples. Glass samples doped with Cu metal have more NBOs (non-bridging oxygen) and are more homogenous than glass doped with CuO, according to the researchers. The glass sample containing copper oxide is different from the other glass since it has the highest optical band-gap value and uses as a narrow-band color filter.
The purpose of the research is the effect of replacing neodymium ions with copper ions (as metal or oxide) on the physical and optical properties of lithium borate glass.
The effect of integrating Cu or CuO into the glass at different concentrations investigates using optical absorption, photoluminescence, thermal analysis, and experimental FTIR findings. The Judd–Ofelt spectral coefficients (Ωλ = 2, 4, 6) utilize to assess the effect on the degree of covalent bonding and stiffness of the Nd3+ ionic sites and the increased 1050 nm luminescence.
2 Experimental work
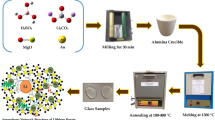
Samples of [80 Li2B4O7-(20-x) Nd2O3-x Cu metal or x CuO mol%] where x = 0, 5, 10, 15, and 20 mol% prepared. Glass samples labeled as 0NdCuO, 5NdCuO, 10NdCuO, 15NdCuO, 20NdCuO, 5NdCu, 10NdCu, 15NdCu, and 20NdCu. The chemicals were weighed and then melted in a porcelain crucible at 1100 °C for 1 h in an electrically heated furnace, (type VAF15/10 Lenton thermal designs). The molted materials quenched in the air.
X-Ray Diffraction (XRD) patterns for samples under study were measured with a Philips Analytical X-Ray diffraction system, type PW3710 based on a Cu tube anode. The samples were ground into a fine powder and then exposed to X-rays radiation with a Cu Kα = 1.54°A (Kα1 = 1.5406°A and Kα2 = 1.54439°A). The XRD data acquire at room temperature using a step scan interval of 0.020° and a step time of 10 s. All samples scan between 10° and 70° (2θ range).
The glass transition, crystallization, and melting temperatures determine using differential thermal analysis (DTA) [Shimadzu DTA 50 thermal analyzer] at a heating rate of 25 °C/min from 25 to 900 °C.
The emission spectra of the samples were measured using a spectrofluorometer (Edinburgh instruments FS5 UK) in wavelengths: UV, visible, and NIR up to 1600 nm. Wavelength accuracy (excitation/Emission) is ± 0.5 nm.
The absorption and transmission spectra of samples were measured with a double-beam V570 JASCO spectrophotometer in a wide range of wavelengths: UV, visible, and NIR from 190 to 2500 nm at 22 °C (room temperature).
The density of glass samples (ρ) determines using the Archimedes method. The density is determined using the following equation, where Wa and Wb represent the mass of glass samples in air and toluene, respectively, and 0.865 g cc is the density of toluene (99.99% purity and use as an immersion liquid).
\(\rho = \, \left( {0.865Wa} \right)/ \, \left( {Wa\, - \,Wb} \right) \, g/cm^{3}\) (or) \({\text{g}}/{\text{cc}}\).
3 Results and discussion
3.1 XRD
Figure 1 shows the X-ray diffraction pattern for glass samples. It depicts a hump with no sharp peaks, thus, indicating that all samples under investigation are in a glassy state.
3.2 Differential thermal analysis DTA
Thermal parameters are significant because they describe the glass structure. Glass-forming ability and thermal stability depend on the creation of bridging and non-bridging oxygen (BOs and NBOs), which is explained by Tg values.
Figure 2 depicts the DTA curves of the samples under investigation. Table 1 evaluates and includes the glass transition (Tg), crystallization (Tc), melting (Tm), and liquid temperatures (Tl).
As demonstrated in Fig. 2 and Table 1, the values of Tg and Tm decrease as the amount of copper metal or copper oxide increases. All Tg, Tc, Tx, and Tm parameters increase linearly with the increase of Nd ions. Tg values decrease, indicating the formation of non-bridging oxygen BO3 [23,24,25]. The open structure and amorphous increase as the number of BOs decreases. Using the equation ΔX = Tc−Tg, Table 1 illustrates the calculated glass-forming ability (GFA) values for the samples under examination (Tc−Tg).
With the addition of copper content, ΔX rises. The temperature difference between the two temperatures Tc and Tg indicates thermal stability and the delay in the nucleation process.
These increases indicate that the glass samples under investigation are unlikely to be influenced by a wide temperature range and are resistant to the production of crystallization phases, implying high-glass composition ability.
J. Jiusti et al. [26] identified some glass stability coefficients (GS) such as Kw, KH, H’, Trg, and Kcr. It can forecast the GFA of oxide glass [27,28,29]. The Weinberg parameter Kw = (Tc−Tg)/Tl came out on top, followed by Hruby [30] is Kh = (Tc−Tg)/(Tm−Tx). The parameter Lu-Liu [31, 32] γ = Tc/(Tg + Tl), as well as the Du and Huang parameters [28] Kcr = (Tl−Tc)/(Tl−Tg), ΔTrg = (Tc−Tg)/(Tl−Tg). Saad and Polan make advantage of [24] (Tc−Tg)/Tg = H’ parameter H’.
Hence, ΔTrg and ΔX each include only one aspect of GFA (either the ease of glass formation or the thermal stability of glass) and cannot measure GFA effectively. Other parameters calculate the glass-forming ability [33,34,35].
Furthermore, they found that using the maximum crystallization temperature Tc rather than the beginning Tx increased the prediction power of these parameters.
3.3 Optical properties
The transmission spectra of [80Li2B4O7-(20−x) Nd2O3-x Cu metal or x CuO mol % samples, where x = 0, 5, 10, 15, and 20 mol%, are shown in Fig. 3.
Figure 3 illustrates the neodymium transition peak, which becomes less intense with copper added, and the glass sample used as a filter cutoff glass. The cutoff increases with the increase of copper oxide from a glass sample containing 10 mol % CuO up to a glass sample containing 20 mol % CuO ranges from 942 nm, 978 nm, and 1406 nm, and for a glass-containing copper metal effect in cutoff ranges from 954 nm, 1028 nm, 1154 nm, and 1282 nm. They also noticed that as copper concentration grew as oxide or metal, the peak intensity increased, and the copper peak overtook the neodymium peak and vanished. Many researchers have looked at the optical characteristics of cu+ in glass [11, 36,37,38].
Figure 3 appears that the addition of copper oxide or copper metal formed wide bands that overlap most of the neodymium band.
Figure 4 shows the optical absorption spectrum of lithium borate glass free from copper oxide or copper metal. The absorption band corresponding to the Nd3+ transition from the ground state 4I9/2 to the excited state (4D5/2,4D3/2), 2P1/2, (2K15/2 + 2G9/2), 4G9/2, 4G7/2, 4G5/2, 2H11/2, 4F9/2, (4F7/2 + 4S3/2),4F5/2, and 4F3/2, at 870, 804, 748, 684, 626, 586, 526, 512, 472, 428, 356, and 330 nm, respectively [39]. The oscillator strength is calculated experimentally using the energy transitions according to the relation [40]:
where NA is the Avogadro number and ∈ (ν) is the molar Absorption coefficient. This equation reduces to
∈ (ν) calculated using the Beer-Lambert Law [25]
where c is the concentration of rare-earth ions, l is the thickness of the sample (cm), and log (I0/I) is the absorption (A).
3.4 3.4 Judd–Ofelt parameters
The oscillator strength determined for an electric dipole transition within 4fn configurations derived using the Judd–Ofelt theorem in terms of the three intensity parameters Ω2, Ω4, and Ω6, according to the expression [22]:
where \({\boldsymbol{\Omega }}_{{\varvec{t}}}\) is the intensity parameter, h is Planck's constant, \(\parallel {\bigcup }^{{\varvec{\lambda}}}\parallel \) there are doubly reduced matrix elements due to the J–J’ transition of Nd3+, \({{\varvec{\chi}}}_{{\varvec{E}}{\varvec{D}}}\) is tensor operators \({(\chi }_{ED}=(\frac{{{(n}^{2}+2)}^{2}}{9n})\), and n is the refractive index. The following equation was used to calculate it:
Tauc plots, as shown in Fig. 5, can use to calculate the optical band gap Eg, which is reported in Table 2.
According to the relationship, the excellent fit between experimentally acquired findings and theoretically derived results from the root-mean-square equation:
where N is the number of energy levels. The small δrms represents an agreement between the experimental and calculated \({f}_{exp}\) and \({f}_{cal}\) (Table 3).
The bonding parameter (δ) determines whether the link between oxygen and Nd is covalent or ionic. The relationship is covalent if the δ value is positive, and vice versa. The Nephelauxetic ratio ß computes the bonding parameter.
It calculates using the formula ß = υc/υa. The wavenumber of a transition for an Nd in the lithium tetraborate is υc and in the aqua ion is υa. The value of δ is calculated using = (1−β’)/β’ and β’ is the average value of β [(β’ = β/x) −x is the number of energy levels]. For the current samples, the value of δ was -0.0146, 0.162, 0.189, and 0.193.
The δ of the neodymium-free sample has a negative value, implying that the Nd3+ bond is ionic. It becomes a covalent bond as the concentration of copper in the samples rises. These findings matched Judd–Ofelt parameters.
The JO intensity parameters could be affected by any changes in the host material. The bonding, symmetry, and rigidity of host matrices are described by the Judd–Ofelt parameter.
The values of Ωλ for samples under investigation are calculated and tabulated in Table 4.
Any change in the host material could affect the JO intensity parameters. The Judd–Ofelt parameter describes the symmetry and rigidity of the host glass sample.
The parameter Ω2 reflects the asymmetry and covalency of the RE ion [41, 42].
The bulk characteristics and rigidity of the produced glasses determine the Ω4 and Ω6 parameters [40].
Table 4 shows the Ω2 value increases as the concentration of Nd3+ in glass doped with copper oxide decreases. The glass samples become more asymmetric, with a high covalent connection between Nd3+ ions and the surrounding oxygen [22, 43].
When compared to parameters (Ω2, Ω4, Ω6) of a sample containing the same concentration of copper oxide, the samples containing copper metal have a lower value of Ω2 and greater values of Ω4, Ω6.
The covalency of the RE3+–O2 bond connects to the Ω6 parameter, which decreases with increasing covalency [44].
The spectroscopic quality factor (χ = Ω4/Ω6)[45] abbreviates the importance of the ratio of Ω4 to Ω6 parameters in determining the emission intensity 4F3/2 → 4I11/2 and 4F3/2 → 4I9/2 transitions of Nd3+.
When Ω4 < Ω6 (the most intense transition is 4F3/2 → 4I11/2), but when Ω4 > Ω6 (the most intense transition is 4F3/2 → 4I9/2) [45,46,47].
According to Table 4, the samples under study tended Ω6 > Ω4 to project the 4F3/2 → 4I11/2 emission intensity.
The JO parameters AESA used to measure the ratio of emission intensity (Aem) to excited-state absorption intensity are given by
If the ratio \(\frac{{{\varvec{A}}}_{{\varvec{e}}{\varvec{m}}}}{{{\varvec{A}}}_{{\varvec{E}}{\varvec{S}}{\varvec{A}}}}\) is greater, then one indicates the possibility of amplification.
3.5 Radiative properties
Based on Judd–Ofelt parameters, the radiative properties for the electric dipole transition as the radiative transition probability (\({A}_{R}\)) express as follows:
Total radiative transition probability (AT), fluorescence branching ratio (βR), and a radiative lifetime of the emitting level [14, 48] are determined and tabulated in Table 5.
Table 5 shows that the samples containing 0 and 5 mol% CuO have a high emission probability, branching ratio, and cross section at 4F3/2 → 4I11/2, indicating that they are appropriate for laser application [14].
Figure 6 shows the LiBONdCu glass’s emission spectra after being excited at 585 nm. Three emission bands at 1347, 1071, and 922 nm are seen, corresponding to the 4F3/2 → 4I13/2, 4F3/2 → 4I11/2, and 4F3/2 → 4I9/2 transitions, respectively. Figure 6 clears that the addition of copper metal or oxide reduces the emission intensity. Otherwise, the peaks at 900 and 1300 nm vanish at samples containing 5 and 10 mol% copper metal.
The stimulated emission cross section, σλp, for the stimulated emission for a fluorescent level is given by [48].
where ∆λeff is the bandwidth and (λP) is the peak wavelength.
The transition 4F3/2 → 4I11/2 centered at 1063 nm has the highest intensity of all the peaks, outnumbering the other two. The emission peak intensity is affected by the concentration of Nd2O3 ions, as seen in Fig. 6. The strength of the emitted peaks diminishes as the concentration of Cu ions rises, indicating a concentration quenching effect. The concentration of Nd3+ ions and the distance between Nd3+–Nd3+ were estimated and are listed in Table 2 based on density measurements.
Table 2 shows that increasing the concentration of Cu ions reduces the distance between Nd3+–Nd3+ ions, increasing the contact between them.
Through cross-relaxations, the increased interaction of Nd3+–Nd3+ ions drives energy migration between the active ions [49]. The strength of the peaks is determined by the interionic gap between Nd3+–Nd3+ ions and the concentration of Nd3+ ions. Concentration quenching is observed by a drop in peak intensity and a cross-relaxation process [50].
Furthermore, as derived from Table 2, the emission peak originating from the 4F3/2 → 4I11/2 state at 1063 nm wavelength has a lot of significance as a potential laser emission wavelength (results from absorption peaks).
Table 6 lists the branching ratios for both experimental (exp) and radiative (R) branches. Table 6 shows that the 4F3/2 → 4I11/2 transition has exp and R values larger than 0.50.
A branching ratio greater than 0.5 suggests that the conditions are conducive to efficient lasing action. Then the glass samples that are devoid of copper oxide and those that include copper oxide are both suitable for producing potential NIR laser emission at 1060 nm.
4 Conclusion
The following compositions were used to make glass samples: x CuO mol % or x Cu metal—80 Li2B4O7-(20−x) Nd2O3, where x = 0, 5, 10, 15, and 20 mol %. The Tg values fall as the copper content increases, indicating that the glass samples become more compact.
For glass samples containing 10 mol % copper oxide, the Judd–Ofelt parameters have a higher value of Ω2, indicating that the glass sample has a high covalent character between Nd3+ and oxygen. For glass samples, the ratio of emission intensity (Aem) to excited-state absorption intensity (AESA) is more than one (Aem/AESA > 1), indicating that amplification can occur.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
A. Herrera, F. Londoño, N.M. Balzaretti, Opt. Mater. (Amst). 113, 110884 (2021)
S.J. Huang, Y.B. Xiao, J.L. Liu, Y. Ji, L.Y. Mao, W.C. Wang, J. Non. Cryst. Solids. 518, 10–17 (2019)
C.D.S. Bordon, E.S. Magalhaes, D.M. da Silva, L.R.P. Kassab, C.B. de Araújo, Opt. Mater. (Amst). 109, 110342 (2020)
I. Kashif, A. Ratep, Mater. Sci. Eng. B. 275, 115488 (2022). https://doi.org/10.1016/j.mseb.2021.115488
N. Deopa, A.S. Rao, J. Lumin. 192, 832–841 (2017). https://doi.org/10.1016/j.jlumin.2017.07.052
V. Bhatia, D. Kumar, A. Kumar, Vl. Mehta, S. Chopra, A. Vij, S.M.D. Rao, S.P. Singh, J. Mater. Sci. Mater. Electron. 30, 677–686 (2019). https://doi.org/10.1007/s10854-018-0336-y
A. Bhogi, P. Kistaiah, J. Aust. Ceram. Soc. 56, 127–138 (2020)
J. Rajagukguk, B. Fitrilawati, J.K. Sinaga, Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 223, 117342 (2019). https://doi.org/10.1016/j.saa.2019.117342
R.S. Gedam, D.D. Ramteke, Trans. Indian Inst. Met. 65, 31–35 (2012)
S.R. Narisimsetti, M. Rajesh, G. RajasekharaReddy, B.S. DevaPrasadRajuDanapandian, Opt. Mater. 97, 109360 (2019). https://doi.org/10.1016/j.optmat.2019.109360
I. Kashif, A. Ratep, Appl. Phys. A Mater. Sci. Process. 120, 1427–1434 (2015). https://doi.org/10.1007/s00339-015-9329-3
C. Tian, X. Chen, Y. Shuibao, Solid State Sci. 48, 171–176 (2015)
F.B. Costa, K. Yukimitu, L.A.O. Nunes, M.S. Figueiredo, L.H.C. Andrade, S.M. Lima, J.C.S. Moraes, J. Phys. Chem. Solids. 88, 54–59 (2016)
K. Linganna, R. Narro-García, H. Desirena, E. De la Rosa, Ch. Basavapoornima, V. Venkatramu, C.K. Jayasankar, J. Alloys Compd. 684, 322–327 (2016). https://doi.org/10.1016/j.jallcom.2016.05.082
V.R. Kumar, N. Purnachand, B.N.K. Reddy, V.R. Kumar, Y. Gandhi, B.V. Ragavaiah, Phys. B Condens. Matter. 600, 412519 (2021)
R.A. Satten, J. Chem. Phys. 21, 637–648 (1953)
E. Snitzer, Phys. Rev. Lett. 7, 444 (1961)
A.L. Dawar, V. Mehta, A. Mansingh, RAJRUP. J. Mater. Sci. Lett. 16, 791–794 (1997)
C.N. Raju, C.A. Reddy, S. Sailaja, H.J. Seo, B.S. Reddy, J. Mater. Sci. 47, 772–778 (2012). https://doi.org/10.1007/s10853-011-5853-5
G. Gupta, A.D. Sontakke, P. Karmakar, K. Biswas, S. Balaji, R. Saha, R. Sen, K. Annapurna, J. Lumin. 149, 163–169 (2014)
P. Pascuta, R. Stefan, L.E. Olar, L.C. Bolundut, E. Culea, Materials (Basel). 13, 1–11 (2020). https://doi.org/10.3390/ma13215040
I. Kashif, A. Ratep, J. Mol. Struct. 1102, 1–5 (2015). https://doi.org/10.1016/j.molstruc.2015.07.070
A.A. Soliman, E.M. Sakr, I. Kashif, Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 158, 30–34 (2009). https://doi.org/10.1016/j.mseb.2008.12.034
I. Kashif, A.A. Soliman, E.M. Sakr, A. Ratep, Phys. Chem. Glas. J. Glas. Sci. Technol. Part B 55, 34–40 (2014)
G. Lakshminarayana, M.K. Kawa, S.O. Baki, A. Lira, A.N. Meza-Rocha, C. Falcony, U. Caldiño, I.V. Kityk, A. Méndez-Blas, A.F. Abas, M.T. Alresheedi, M.A. Mahdi, Opt. Mater. (Amst). 78, 142–159 (2018). https://doi.org/10.1016/j.optmat.2018.02.011
J. Jiusti, D.R. Cassar, E.D. Zanotto, Int. J. Appl. Glas. Sci. 11, 612–621 (2020)
G.-H. Zhang, K.-C. Chou, J. Appl. Phys. 106, 94902 (2009)
X.H. Du, J.C. Huang, Chinese Phys. B 17, 249 (2008)
M. Saad, M. Poulain, Mater. Sci. Forum 19, 11–18 (1987)
A. Hruby, Czechoslov. J. Physl. 22, 1187–1193 (1972)
L. Lu ZP, Acta. Mater. 50, 3501–3512 (2002)
Z.P. Lu, C.T. Liu, Intermet. 12, 1035–1043 (2004)
G.N. Greaves, S. Sen, Adv. Phys. 56, 1–166 (2007)
K. Mondal, B.S. Murty, J. Non. Cryst. Solids. 351, 1366–1371 (2005)
I. Kashif, E.M. Sakr, A.A. Soliman, A. Ratep, Phys. Chem. Glas. J. Glas. Sci. Technol. Part B 54, 35–41 (2013)
J.A. Jiménez, J. Mater. Sci. 49, 4387–4393 (2014). https://doi.org/10.1007/s10853-014-8138-y
J.A. Jiménez, J. Phys. Chem. Solids. 75, 1334–1339 (2014). https://doi.org/10.1016/j.jpcs.2014.07.005
J.A. Jiménez, M. Sendova, Mater. Chem. Phys. 162, 425–430 (2015). https://doi.org/10.1016/j.matchemphys.2015.06.009
A.U. Ahmad, S. Hashim, S.K. Goshal, Optik (Stuttg). 199, 163515 (2019). https://doi.org/10.1016/j.ijleo.2019.163515
S. Mohan, K.S. Thind, D. Singh, L. Gerward, Glas. Phys. Chem. 34, 265–273 (2008)
N.B. Shigihalli, R. Rajaramakrishna, R.V. Anavekar, Can. J. Phys. 91, 322–327 (2013). https://doi.org/10.1139/cjp-2012-0062
Y. Qiao, N. Da, D. Chen, Q. Zhou, J. Qiu, T. Akai, Appl. Phys. B Lasers Opt. 87, 717–722 (2007). https://doi.org/10.1007/s00340-007-2637-3
M. Farouk, A. Abd El-Maboud, M. Ibrahim, A. Ratep, I. Kashif, Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 149, 338–342 (2015)
B.V. Padlyak, R. Lisiecki, T.B. Padlyak, V.T. Adamiv, J. Lumin. 198, 183–192 (2018). https://doi.org/10.1016/j.jlumin.2018.02.046
A. Kumar, D.K. Rai, S.B. Rai, Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 58, 2115–2125 (2002)
J.S. Kumar, K. Pavani, S.R. Gavinho, M. Seshadri, V. Anjos, M.J.V. Bell, F.N. Freire, M.A. Valente, M.J. Soares, M.P.F. Graça, J. Non. Cryst. Solids. 498, 89–94 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.05.031
B. Karthikeyan, R. Philip, S. Mohan, Opt. Commun. 246, 153–162 (2005). https://doi.org/10.1016/j.optcom.2004.10.051
F. Zaman, N. Srisittipokakun, G. Rooh, S.A. Khattak, N. Singkiburin, H.J. Kim, N. Sangwaranatee, J. Kaewkhao, J. Lumin. 215, 116639 (2019). https://doi.org/10.1016/j.jlumin.2019.116639
C.R. Kesavulu, H.J. Kim, S.W. Lee, J. Kaewkhao, N. Chanthima, Y. Tariwong, J. Alloys Compd. 726, 1062–1071 (2017)
R. Rajaramakrishna, B. Knorr, V. Dierolf, R.V. Anavekar, H. Jain, J. Lumin. 156, 192–198 (2014). https://doi.org/10.1016/j.jlumin.2014.07.021
Acknowledgements
I.Kashif thanks Prof. Dr. A. M. Sanad for his support throughout my scientific career during his stay with us.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
AR: Investigation, Writing—original draft, Methodology, Formal analysis. IK: Writing—review & editing, administration, Formal analysis, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
This paper meets the ethical standards of this journal.
Consent to participate
All authors agree with the review of this paper in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kashif, I., Ratep, A. The effect of replacing copper metal or oxide with neodymium on the optical properties of lithium tetraborate glass. J Mater Sci: Mater Electron 33, 19231–19241 (2022). https://doi.org/10.1007/s10854-022-08761-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08761-1