Abstract
Polyvinylidene fluoride (PVDF) polymer is considered as an encouraging piezoelectric material whose optical properties need to be enhanced. Zinc ferrite is an excellent photoelectric material, in the present work it was doped separately by (cobalt and copper) ferrite. Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles were synthesized using auto-combustion method and characterized using XRD, FTIR, FESEM and HRTEM. The crystallite size was (28.8, 35.8, 22 and 31) nm for the Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles, while the estimated particle size from HRTEM were 55 nm for the Co-ZnFe2O4 nanoparticles and 75 nm for the Cu-ZnFe2O4. The obtained samples used as PVDF fillers, aiming to improve its optical properties. The optical properties as well as, the piezoelectric response of the prepared PVDF/(Co-ZnFe2O4 and Cu-ZnFe2O4) nanocomposites were investigated. The maximum value for refractive index was related to PVDF/Co-ZnFe2O4 nanocomposite. While, Cu-ZnFe2O4 nanoparticles have limited improvement of the PVDF optical properties. Co-ZnFe2O4 nanoparticles increases the PVDF optical conductivity σopt. four times its original value as well as the dielectric values was increased from 0.05 for neat PVDF to 2. The piezoelectric response of the PVDF polymer is clearly increased by the addition of both Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ferrites are consisting of Fe2O3 and FeO with a general formula of AB2O4; representing the ceramic acquiring ferrimagnetic properties. These properties can be adjusted by doping. The structure of ferrite is related to the ions distribution over tetrahedral (A)-site and octahedral [B]-sites in the lattice structure. They have tunable structural, high electrical, great catalytic activity, as well as decent magnetic properties etc. [1]. Recently, many research areas pay attention to the synthesis and the application of ZnFe2O4 because of its escalating applications and restricted properties. ZnFe2O4 has soft magnetic nature, acquiring a normal spinel phase [2]. It is observed that doping Zn ferrite with magnetic divalent ions such as Ni performs an important role in the improvement of the magnetic properties [3]. In the last decades, there is a possibility of support for using renewable energy, for its economic, environmentally friendly properties. Energy harvesting from mechanical energy (piezoelectricity) become very influential lately. Piezoelectricity is produced due to the spontaneous separation of charge within crystal structures in certain conditions. A displacement of the electron relative to their atomic centers, referred to an electric polarization produced by the application of mechanical stress along the appropriate direction of a crystal, however, an applied electric field can generate a mechanical distortion in the material. The phenomenon of Piezoelectricity can be used in many applications, including sensors and actuator applications. Piezoelectricity is defined as the charge accumulation on the solid material’s surface once the material is exposed to mechanical stress. The piezoelectric substances exhibit numerous potential applications in relation to diverse industries as computer, medical, automotive, consumer etc. [4]
Ceramic piezoelectric materials have a great role in the field of sensors [5]. Although these materials have large relative permittivity, facilitate electrical tuning, they are tainted by defects such as low inherent breakdown strength, these materials are brittle which limits their applications in flexible or wearable devices.
Polyvinylidene fluoride (PVDF) polymer is a good alternative for ceramic materials. It is possessing unique advantages over ceramic such as flexibility, further, PVDF is inert chemically, it has electroactive β-polymorphic phase with great toughness and small fatigue failure can produce remarkable voltage under long-term continuous oscillations. However, PVDF has the disadvantage of low-dielectric permittivity. Coupling between polymer and ceramic fillers nanoparticles gives a composite material with new properties. This modification related to in situ growth of β-crystalline phase within the nanocomposites which improve the dielectric and energy harvesting performance of the nanocomposite [5,6,7]. The increase in dipole–dipole interaction with the increase of the filler content leads to higher dielectric constant composite material [6, 8, 9].
A lot of interest has been paid to the piezoelectric materials for its excellent properties that combine the strength of polymers. Recently, the need for further research in the field of piezotronics and piezophototronics increased, where charge–carrier transport is controlled by the application of external mechanical stimuli in flexible devices [10]. The fundamental investigations of piezoelectricity and semiconductor properties utilizing useful nanomaterials prompts the improvement of more intelligent electronic and optoelectronic materials [11].
In the present study, Co0.5Zn0.5Fe2O4 and Cu0.5Zn0.5Fe2O4 have been prepared, we used these ratios to make the doping effect to be clear. The synthesized samples investigated as PVDF nanofillers, where Co-ZnFe2O4 and Cu-ZnFe2O4 are reported as semiconductor materials [12, 13]. The investigated nanocomposites were prepared using simple solution casting method [14] The PVDF polymer has dissolved in dimethyl formamide (NMP), Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles are synthesized, characterized, and introduced into the solution as the PVDF nanofiller. The effect of Co-ZnFe2O4 compared to Cu-ZnFe2O4 on the PVDF optical properties and piezoelectric response were investigated. The present work aims to study the piezoelectric and optoelectronic properties of PVDF/(Co-ZnFe2O4 and Cu-ZnFe2O4).
2 Experimental
2.1 Preparation of MFe2O4 nanoparticles
Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles were synthesized by citrate auto-combustion rout (Fig. 1). A suitable amount of iron nitrate Fe(NO3)3·9H2O (99%), cobalt nitrate Co(NO3)2·6H2O, copper nitrate Cu(NO3)2·6H2O (99%), zinc nitrate [Zn(NO3)2·6H2O] and citric acid (C6H8O7), in the required stoichiometric ratios mixed together in a small amount of distilled water. Then, the pH of the solution was adjusted at 7. After that, the temperature was raised up to 250 °C until all fumes ended. Finally, the obtained powder was calcined for 4 h at 800 °C.
2.2 Synthesis of PVDF/MFe2O4 nanocomposites
The obtained nanoparticles have been used as the PVDF fillers. Three grams of Poly (vinylidene fluoride) (PVDF) powder (Sigma-Aldrich, USA), powder were dissolved in 10 mL of N-methyl-2-pyrrolidone (NMP, 99.5% of purity) (Merk Chemical, India), at room temperature under continuous stirring for 6 h. to obtain a transparent solution. Another solution of 3 mg of the Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles were dissolved in appropriate amount of (NMP) and sonicated. To prepare the nanocomposite films of 0.01 wt% of nanofillers, the powder solution was added to the (PVDF/NMP) solution and sonicated for 2 h. Finally, each sample was poured on a clean glass surface on a hotplate kept at 60 °C. The obtained films of about (50 × 50 × 0.16 mm) washed using distilled water to remove any contaminating particles and for full solidification of the films.
2.3 Characterization of nanoparticles
Fourier transformed infrared (FTIR) spectra usually is utilized to investigate the bond groups and it was done out by FTIR spectrometer (Perkin-Elmer system 2000) in the range of 4000–400 cm−1. The degree of crystallinity was investigated using, X-ray diffraction (XRD) was carried out via X-ray diffractometer (Analytical-X’ Pertpro, Cu Kα1 radiation, λ = 1.5404 Å, 45 kV, 40 mA, The Netherlands). The collected data in the range of 4° ≤ 2θ ≤ 60°, a step size is 0.02° and a step time of 0.5 s. High-resolution transmission electron microscopy (, JEOL/JME 2100) was used to investigate the morphology and microstructure of the prepared samples. The operating voltage was 200 kV.
2.4 Optical properties investigation
UV–Vis spectra of the prepared nanocomposite films obtained using (JASCO Corp., V-570, Rev. 1.00).
2.5 Piezoelectric response
2.5.1 Digital storage oscilloscope
The created voltage by repeating human finger press and release on the surface of the investigated nanocomposite films has been recorded using a digital storage oscilloscope [GW Instek Gos-806s]. The responses were recorded in terms of open circuit output voltage, at room temperature.
2.5.2 Piezo response force microscopy (PFM)
Flex-Axiom AFM was used for measuring the piezoresponse force. The requirements of the PFM are commercial head type with 115–135 μm length, Co–Cr-coated tip with electrical resistivity of 0.01–0.025 Ω cm with nearly 35 nm tip curvature nominal spring constant of 5 N/m and 165.08 kHz nominal resonance frequency. A.c. voltage was applied to the tip, at a frequency of 165.08 kHz for measuring the piezoresponse of samples. Scan rate was 0.5 Hz while the scan area was (250 × 250) nm2. Insulating chamber is used for all measurements at room temperature.
3 Results
3.1 Nanoparticles characterization
3.1.1 XRD
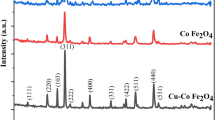
Figure 2a and b illustrates the XRD patterns of the synthesized Co-ZnFe2O4 and Cu-ZnFe2O4 powder. From the figure it can be noted the formation of single-phase cubic spinel and cubic structures for Co-ZnFe2O4 and Cu-ZnFe2O4 samples respectively. The spectrum shown in the figure confirms that the obtained patterns match well the reported standard phase in the XRD reference ICDD cards: [04–002-0421] and [01–077-0013] for Zinc Iron Cobalt Oxide and Copper Zinc Iron Oxide, respectively. Few peaks appeared in the pattern which corresponding to hematite, that is compatible with the ICDD card [00–024-0072]. While Fig. 2c is related to XRD pattern of PVDF/Co-ZnFe2O4 and Cu-ZnFe2O4 nanocomposite films. The figure indicates the presence of PVDF according to the card [01–072-1174]. The peaks diffracted from (100), (020) and (021), at 17.75°, 18.30°, and 26.50° belong to the α-phase. However, peaks at 19.85°, and 35.90° reflected from (110) and (200) are attributed to β- and γ-phases of PVDF polymer. From Fig. 2 it can be noted that β- and γ-phases are more intense in samples containing Cu-ZnFe2O4 nanoparticles. Making PVDF/Cu-ZnFe2O4 nanocomposite more suitable for piezoelectric applications compared to PVDF/Co-Zn Fe2O4 nanocomposite.
The particle sizes of the prepared nanoparticles were calculated using Scherrer’s equation [15]
where D is the crystallite size (nm), K is the particle shape factor (0.9), λ is the target wavelength (nm), β is the corrected full width at half maximum, and θ is the position (angle) at the maximum of the peak at. The estimated crystallite sizes are (28.8, 35.8, 22 and 31) nm for the Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles, PVDF/Co-ZnFe2O4 and PVDF/Cu-ZnFe2O4 nanocomposites respectively. The crystallite size was decreased by adding PVDF polymer as the crystallinity decreased due to the amorphous nature of the polymer material. Furthermore, the lattice constant (a) of the investigated cubic samples were calculated using the following relation:
where d is the inter-planer spacing, and (hkl) are the miller indices. The obtained results were 8.41 Å and 8.42 Å for Cu-ZnFe2O4 and Co-ZnFe2O4 nanoparticles respectively. This is due to the larger ionic radius of cobalt than that of copper [16,17,18,19].
3.1.2 FTIR
FTIR spectra in ferrites could be labelled into two main ranges: 400–450 cm−1, which could be related to the octahedral site (υ1), while 480–600 cm−1, refers to the tetrahedral site (υ2). Absorption bands in the range 4000–400 cm−1 of Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles are shown in Fig. 3. The bands located at 2900–2997 cm−1 related to the O–H stretching bond existing in the adsorbed water molecules. The peaks corresponding to 1117 cm−1 band is attributed to the Fe–Co alloy system [20, 21]. Bands present at 535 -533 cm−1 belongs to the stretching vibrations along the [Metal ↔ O] bond at the tetrahedral (A) site [22]. In contrast, the relatively low wavenumbers of 430 cm−1 and 412 cm−1 denote the stretching vibrations along the [Metal ↔ O] bond at the octahedral (B) position (M denoted to copper or cobalt) [23]. In addition, the band that is observed around 670 cm−1 is assigned to (Fe3O4) [24, 25].
3.1.3 HRTEM
The HRTEM micrographs in Fig. 4a, b of Co-ZnFe2O4 and Cu-ZnFe2O4 respectively reveal that well-defined cubic shapes. The nano-crystallites have cubic spinel structure with average diameter of 55 nm for the Co-ZnFe2O4 nanoparticles and 75 nm for the Cu-ZnFe2O4. The Cu-ZnFe2O4 particles showed more agglomeration where some particles formed large clusters. This variation in particle size as well as agglomeration may induce a significant change in optical and piezoelectric properties.
3.1.4 FESEM
Figure 5a, b micrographs displays the surface morphology of the prepared samples. It is clear that Co-ZnFe2O4 and Cu-ZnFe2O4 nanocomposite have uniform distribution. Figure 5a, b donated the elemental composition of the investigated samples Co-ZnFe2O4 and Cu-ZnFe2O4 (EDX) spectra it can be observed only the elemental peaks of Co, Zn, O and Fe clarifying the single phase of the Co-ZnFe2O4 sample. However, Fig. 6b identifies the elemental peaks of Cu, Zn, O and Fe. Without any other peaks clarifying the purity of the Cu-ZnFe2O4 sample. Elemental compositions % of Co-ZnFe2O4 and Cu-ZnFe2O4 samples are shown in Table [1]. This obtained data indicates that the atomic compositions % of Co-ZnFe2O4 and Cu-ZnFe2O4 samples agreed with the stoichiometric ratio of elements in synthesized nanoparticles (Table 1).
3.2 Optical properties
3.2.1 Reflectance and transmittance
Figure 7 shows the reflectance of UV–Vis spectrum of Co-ZnFe2O4 and Cu-ZnFe2O4. Figure 7 indicates an increase in the reflectance intensity by increasing wavelength.
Figure 8 explains the reflectance intensity of the pure PVDF polymer and prepared nanocomposites. It is obvious that there is clear increase in the PVDF reflectance when nano-fillers were added, the largest values were spotted in case of PVDF/(Co-ZnFe2O4) nanocomposite, this increase reached 5 times the PVDF original value. This observed increase in the nanocomposite reflectance is attributed to light scattering caused by the nanoparticles, where cobalt has higher refractive index [26]. We can conclude that the (PVDF/Co-ZnFe2O4) nanocomposite film had the lower transmittance intensity.
Moreover, Fig. 9a shows a remarkable increase in the PVDF absorption coefficient (α) with the addition of nanofillers. The absorption coefficient (α) of the investigated samples were obtained using the following equation [27].
where A is the absorbance and l is the thickness of the specimen.
The high absorbance values in the UV region for the investigated nanocomposite films make them of interest in UV protection applications [28, 29].
The extinction coefficient (K) was calculated using the following equation [27]
where α and λ are the absorption coefficient, and wavelength, respectively.
The relation between the extinction coefficient (K) and wavelength for neat PVDF and (PVDF/Co-ZnFe2O4) nanocomposite is illustrated in Fig. 9b. It is observed that (K) values increases by increasing the wavelength which attributed to the interaction between the incident photons and electrons. As well as K is increased by the addition of the nanofillers as the density of the PVDF increased by addition of nanofiller.
3.2.2 Optical bandgap
An electron can be moved from its normal state to an excited state as it is absorbing energy from an incident photon greater than its band gap energy. In photoexcitation, an electron moves from the valence band into the conduction band across the optical band gap. The lower the energy of the band gap (\({E}_{\mathrm{g}}\)), the easier for an electron to move from the valance band to the conduction band [30]. In the present work, the optical band gap energy can be estimated using Tauc’s relation (T-region):
where α is absorption coefficient, A is constant, n indicates the optical transition type (n = 2 indicates direct transition and n = ½ indicates indirect transition) [31,32,33]. Figure 10 shows the relation between \({\left(\alpha h\nu \right)}^{2}\) verse the incident photon energy (\(h\nu )\). From the linear parts of the obtained curves the direct band gap of the neat PVDF and PVDF/(Co-ZnFe2O4, Cu-ZnFe2O4) nanocomposites have been calculated and tabulated in Table 2. It is noticed that the addition of nanoparticles, reduced the PVDF band gap to nearly half its original value, which might be due to incorporation lager number of defects leading to the creation of new levels in the PVDF band gap facilitates the movement of electrons from valance to conduction band. The obtained results compete many of recently published works shown in Table 3.
3.2.3 Refractive index
The refractive index is another mainly important elemental property of material because of its direct relationship with the ion’s electronic polarizability and the local field within the material. The refractive index (n) of composite materials has been calculated via given equation [43],
where R is reflectance and K is the extinction coefficient.
Figure 11 shows the plot of the refractive index (n) versus (λ) of the samples under investigation. A remarkable increase in the refractive index value (n) was observed in the PVDF by the addition of the Co-ZnFe2O4 nanoparticles. This increase can be attributed to the higher intermolecular chemical and physical interaction between the filler and the adjacent PVDF chain segments which lead an improvement of the films densities resulting in higher refractive indices and the higher refractive index [26]. The maximum value for refractive index was related to PVDF/Co-ZnFe2O4 nanocomposite.
3.2.4 Optical conductivity
The optical conductivity σopt. is an important parameter for studying the electronic states in materials. The optical conductivity was obtained using the following equation, [27]:
α is the absorption coefficient, and (n) is the refractive index of the samples.
Figure 12 illustrates the variation of optical conductivity σopt. as a function of photon energy hν for the investigated samples. It is observed that the optical conductivity of PVDF increases by the addition of nanoparticles. This increase can be attributed to the creation of new levels in the band gap which facilitates the movement of charges from valance to conduction band [44]. Co-ZnFe2O4 nanoparticles increases the PVDF optical conductivity σopt. four times its original value.
3.2.5 Dielectric constant
The complex dielectric constant reveals an insight into the behavior of electrical charge carriers in materials. The real part of dielectric constant is representing the amount by which the velocity of light decreases within the material, although imaginary part of dielectric constant represents the amount of energy absorbed in the dielectric material from electric field due to dipole motion. Both the real and imaginary parts of dielectric constant have been calculated by using following expressions [27, 45, 46].
where εr is real part of dielectric constant, εi is imaginary part of dielectric constant. The real part of dielectric constant (εr) and imaginary part (dielectric loss) (εi) as a function of (hν) of the investigated samples shown in Fig. 13a, b. It can be noted that the prepared nanoparticles (Co-ZnFe2O4 and Cu-ZnFe2O4) have improved the dielectric response of the PVDF polymer. The greater dielectric values were observed in case of (Co-ZnFe2O4) nanofiller where it increases the dielectric constant from 0.05 for neat PVDF to 2. These greater dielectric values resulted from the interfacial polarizations at the conductor–insulator interface [47,48,49]. As well as the PVDF dielectric loss increases by the addition of the nanoparticles, the higher increase was observed in the PVDF/Cu-ZnFe2O4 sample this increase is attributed to the increase in n and K with the nanofillers.
3.2.6 Non-linear optical (NLO) properties
Studying the nonlinear optics of PVDF/(Cu-ZnFe2O4) nanocomposites is helpful for the usage in several optoelectronic applications [50]. The intensity of incident light causing the occurrence of optical polarizability P in the nanocomposite. The nonlinear electron polarizability PNL can be obtained using the following equations [51]:
where E is the electric field of light, \({\chi }^{(1)}\) is the linear optical susceptibility, \({\chi }^{(2)}\) is the second order nonlinear optical susceptibility and \({\chi }^{(3)}\) is the third order nonlinear optical susceptibility [52,53,54].
To determine the values of, \({\chi }^{(1)}\) and \({\chi }^{(3)}\) these equations can be used
where \(\xi\) = 1.7 × 10–10 (esu). Figure 14a, b indicates the obtained values of \({\chi }^{(1)}\) and \({\chi }^{(3)}\) as a function of wavelength for pure PVDF and PVDF/(Co-ZnFe2O4 and Cu-ZnFe2O4) nanocomposites. For pure PVDF and PVDF/(Cu-ZnFe2O4) nanocomposite samples, the variations of \({\chi }^{(1)}\) and \({\chi }^{(3)}\) are very small. The PVDF and PVDF/(Cu-ZnFe2O4) values are almost constant and have the same pattern for both \({\chi }^{(1)}\) and \({\chi }^{(3)}.\) Moreover, it is observed that the Co-ZnFe2O4 nanofiller values of \({\chi }^{(3)}\) are increased and improved the nonlinear response of the PVDF polymer.
3.3 Energy harvest performance
Piezoelectricity is an electric polarization proportional to an applied mechanical energy or a mechanical deformation proportional to an applied electric field.
3.3.1 Piezoelectric response using a digital storage oscilloscope (DOS)
The working principle of the piezoelectric energy harvester of PVDF is based on the creation of an electric polarization under an applied stress [55].
The prepared nanocomposite films have been tested using a digital storage oscilloscope (DOS), where each film was placed between two copper layer electrodes. With respect to the open circuit (with forward and reverse connection), the generated piezo potential was recorded as a repetitive finger stress has been applied on the upper surface of nanocomposite films. The finger tapping generates a compressive stress on the surface of films, causes the displacement of positive and negative charges in the nanocomposite films.
It is well known that PVDF mainly have a synthetic semi crystalline polymer, and its β-phase and γ-phases are responsible for the piezoelectric power harvesting property [5].
By mechanical deformation and polarization, the structure of alpha phase can be transformed into polar beta phase to achieve piezoelectric characteristics [56].
The filler nanoparticles improved the piezo-potential as shown in Fig. 15a–c where an interaction between the Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles and the dipoles of PVDF (CH2 = CF2).
This enhancement of piezo-potential in PVDF composite films is due to the role of Co-ZnFe2O4 and Cu-ZnFe2O4 nanofillers in PVDF matrix, which is provide a conducting particle could help charge carriers induced inside the film to move to the surface. In addition, the interaction between Co2+, Zn2+, Cu2+ and Fe3+ nanoparticles with CF2-dipoles and O2− nanoparticles of Co-ZnFe2O4 and Cu–Zn Fe2O4 with CH2-dipoles could enhance the piezo response of PVDF polymer.
It can be noted that the nanofillers have enhanced the values of complex dielectric constant and the values of piezo potential of the PVDF polymer as shown in Figs. 12 and 14. That could be due to the increase in polarization causes a slight increase in the dielectric constant, where the beta phase dipoles rearranged and a transformation of alpha to beta takes place when energy is applied [57].
3.3.2 PFM studies
Piezoresponse force microscopy (PFM) was used for measuring piezoelectric response of prepared nano samples. PFM operated in contact mode using an alternating voltage Va.c. applied to the tip subsequent in an alternating electric field inside the specimen [58, 59]. Herein, piezoelectric samples, a periodic deformation was applied on the samples. The applied deformation causes deflection of cantilever in any directions; torsion or buckling [60]. Plane deformation (a change in the z-axis) was causing the deflection, buckling responds to an in-plane deformation (a diameter changes in the y-axis) as well as torsion is related to another in-plane deformation (a length change in the x-axis) [61]. Resolved lateral mapping of the piezoelectric behavior of the prepared sample can be obtained using PFM. The combination between PFM images of in-plane and out-of-plane which are obtained from PFM are related to domain structure [62]. The area of 2.5 µm × 2.5 µm of the pure PVDF and PVDF/(Co-ZnFe2O4, Cu-ZnFe2O4,) nanocomposite films was scanned by using Va.c. of 510 mV applied to the cantilever tip. The response of PFM in the z-axis direction, phase, and amplitude micrographs of the considered samples, respectively is shown in Fig. 16. The out of plane PFM phase pictures (Fig. 17) for all tested samples, corresponding to the piezoelectric polarization, both negative (white) and positive (black) zones showed up representing antiparallel ferroelectric nanodomains with 180° domain walls [63, 64]. The white zones related to negative domains with the polarization direction perpendicular to the surface of the PVDF film and Orien situated descending, whereas the dark areas associated with positive domains having the polarization heading upward [65].
In pure PVDF, the well-defined piezoelectric spaces illustrate that the stretched crystallites are the homogeneous β-phase. The PFM amplitude for PVDF/(Co-ZnFe2O4, Cu-ZnFe2O4,) nanocomposite films (a piezoelectric contrast due to the defections caused by the applied alternating field has been noted. Therefore, the domains in the PVDF film align along the z-axis direction perpendicular to the sample and the bottom electrode, Fig. 17. The in-plane PFM images (Fig. 16d–j) were analyzed both for the phase and amplitude dependence of the PFM signal of PVDF/(Co-ZnFe2O4, Cu-ZnFe2O4) nanocomposites films. Figures 16 and 17 donate the formation of periodic stripe domains acquired at θ = 0°. Stripe domains aligned along the last saturating field direction were observed for all the pictures of PVDF/(Co-ZnFe2O4, Cu-ZnFe2O4) nanocomposites films.
4 Conclusions
Copper doped zinc ferrite- and cobalt-doped zinc ferrite nanoparticles were successfully synthesized and characterized using different characterization methods. The prepared Co-ZnFe2O4 and Cu-ZnFe2O4 nanoparticles have been used as fillers of the PVDF polymer.
The optical properties as well as piezoelectric response of pure PVDF and PVDF/(Co-ZnFe2O4 and Cu-ZnFe2O4) nanocomposites were studied. The obtained results showed a great development in the relative permittivity, optical conductivity, refractive index, and non-linear susceptibility of the PVDF/(Co-ZnFe2O4) nanocomposite, where PVDF/(Cu-ZnFe2O4) nanocomposite showed a lower improvement in the previous optical properties.
Both PVDF/(Co-ZnFe2O4 and Cu-ZnFe2O4) nanocomposites had lower band gap energy values than that of the pure PVDF, makes them convenient for opto-electronic applications.
The two prepared nanocomposites improved the PVDF piezoelectricity, the PVDF/(Co-ZnFe2O4 and Cu-ZnFe2O4) nanocomposites films show stripe domains aligned along the last saturating field direction when examined using PFM., the domains in the PVDF film align along the z-axis direction perpendicular to the sample and the bottom electrode.
From the obtained results, we can say that PVDF/(Co-ZnFe2O4) nanocomposite is a great candidate for piezo-phototropic applications.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
M.A. Almessiere, Y. Slimani, A. Demir Korkmaz, S. Güner, A. Baykal, S.E. Shirsath, I. Ercan, P. Kögerler, Sonochemical synthesis of Dy3+ substituted Mn0.5Zn0.5Fe2−xO4 nanoparticles: structural, magnetic and optical characterizations. Ultrason. Sonochem. 61, 104836 (2020)
P. Annie Vinosha, A. Manikandan, R. Ragu, A. Dinesh, P. Paulraj, Y. Slimani, M.A. Almessiere, A. Baykal, J. Madhavan, B. Xavier, G. Francisco Nirmala, Exploring the influence of varying pH on structural, electro-optical, magnetic and photo-Fenton properties of mesoporous ZnFe2O4 nanocrystals. Environ. Pollut. 272, 115983 (2021)
M.A. Almessiere, Y. Slimani, A.V. Trukhanov, A. Demir Korkmaz, S. Guner, S. Akhtar, S.E. Shirsath, A. Baykal, I. Ercan, Effect of Nd–Y co-substitution on structural, magnetic, optical and microwave properties of NiCuZn nanospinel ferrites. J. Mater. Res. Technol. 9(5), 11278–11290 (2020)
S. Ramesh, D. Ravinder, K.C. Babu Naidu, N.S. Kumar, K. Srinivas, D. Baba Basha, B. Chandra Sekhar, A review on giant piezoelectric coefficient, materials and applications. Biointerface Res. Appl. Chem. 9, 4205–4216 (2019)
A.M. AlAhzm, M.O. Alejli, D. Ponnamma, Y. Elgawady, M.A.A. Al-Maadeed, Piezoelectric properties of zinc oxide/iron oxide filled polyvinylidene fluoride nanocomposite fibers. J. Mater. Sci. Mater. Electron. 32(11), 14610–14622 (2021). https://doi.org/10.1007/s10854-021-06020-3
M.A. Almessiere, Y. Slimani, M. Sertkol, F.A. Khan, M. Nawaz, H. Tombuloglu, E.A. Al-Suhaimi, A. Baykal, Ce–Nd co-substituted nanospinel cobalt ferrites: an investigation of their structural, magnetic, optical, and apoptotic properties. Ceram. Int. 45(13), 16147–16156 (2019)
L. Yang, J. Qiu, H. Ji, K. Zhu, J. Wang, Enhanced dielectric and ferroelectric properties induced by TiO2@MWCNTs nanoparticles in flexible poly(vinylidene fluoride) composites. Compos. A Appl. Sci. Manuf. 65, 125–134 (2014). https://doi.org/10.1016/j.compositesa.2014.06.006
K. Sappati, S. Bhadra, Piezoelectric polymer and paper substrates: a review. Sensors 18(11), 3605 (2018). https://doi.org/10.3390/s18113605
T.K. Sinha, S.K. Ghosh, R. Maiti, S. Jana, B. Adhikari, D. Mandal, S.K. Ray, Graphene-silver-induced self-polarized PVDF-based flexible plasmonic nanogenerator toward the realization for new class of self-powered optical sensor. ACS Appl. Mater. Interfaces 8(24), 14986–14993 (2016). https://doi.org/10.1021/acsami.6b01547
W. Wu, Z.L. Wang, Piezotronics and piezo-phototronics for adaptive electronics and optoelectronics. Nat. Rev. Mater. (2016). https://doi.org/10.1038/natrevmats.2016.31
M.M. Saikh, N.A. Hoque, P. Biswas, W. Rahman, N. Das, S. Das, P. Thakur, Self-polarized ZrO2/poly (vinylidene fluoride-co-hexafluoropropylene) nanocomposite-based piezoelectric nanogenerator and single-electrode triboelectric nanogenerator for sustainable energy harvesting from human movements. Physica Status Solidi (A) 218(9), 2000695 (2021). https://doi.org/10.1002/pssa.202000695
D. Ravinder, Thermoelectric power studies of zinc substituted copper ferrites. J. Alloys Compd. 291(1–2), 208–214 (1999). https://doi.org/10.1016/s0925-8388(99)00287-x
S. Supriya, S. Kumar, M. Kar, Band gap engineering of zinc substituted cobalt ferrite for optoelectronic applications. In: 2017 IEEE 12th Nanotechnology Materials and Devices Conference (NMDC), October 2017. https://doi.org/10.1109/nmdc.2017.8350491
M. Haponska, A. Trojanowska, A. Nogalska, R. Jastrzab, T. Gumi, B. Tylkowski, PVDF membrane morphology—influence of polymer molecular weight and preparation temperature. Polymers 9(12), 718 (2017). https://doi.org/10.3390/polym9120718
M.A.R. Miranda, J.M. Sasaki, The limit of application of the Scherrer equation. Acta Crystallogr. Sect. A Found. Adv. 74(1), 54–65 (2018). https://doi.org/10.1107/s2053273317014929
N. Raghuram, T. Subba Rao, K. Chandra Babu Naidu, Ceram. Int. 46, 5894 (2020)
N.S. Kumar, R.P. Suvarna, K. Chandra Babu Naidu, Cryst. Res. Technol. 53, 1800139 (2018)
S. Dastagiri, M.V. Lakshmaiah, K. Chandra Babu Naidu, N. Suresh Kumar, A. Khan, J. Mater. Sci. Mater. Electron. 30, 20253 (2019)
D. Kothandan, R. Jeevan Kumar, K. Chandra Babu Naidu, J. Asian Ceram. Soc. 6, 1 (2018)
S. Rana, J. Philip, B. Raj, Micelle based synthesis of cobalt ferrite nanoparticles and its characterization using Fourier transform infrared transmission spectrometry and thermogravimetry. Mater. Chem. Phys. 124(1), 264–269 (2010). https://doi.org/10.1016/j.matchemphys.2010.06.029
D. Karthickraja, S. Karthi, G.A. Kumar, D.K. Sardar, G.C. Dannangoda, K.S. Martirosyan, E.K. Girija, Fabrication of core–shell CoFe2O4@HAp nanoparticles: a novel magnetic platform for biomedical applications. New J. Chem. 43(34), 13584–13593 (2019). https://doi.org/10.1039/c9nj02510c
R. Ramadan, Physical study of cobalt ferrite and its application in purification of water. Appl. Phys. A (2019). https://doi.org/10.1007/s00339-019-3121-8
N.B. Singh, K. Rachna, Copper ferrite-polyaniline nanocomposite and its application for Cr (VI) ion removal from aqueous solution. Environ. Nanotechnol. Monit. Manag. 14, 100301 (2020). https://doi.org/10.1016/j.enmm.2020.100301
K. Tedjieukeng, H. Mathias, P.K. Tsobnang, R.L. Fomekong, E.P. Etape, P.A. Joy, A. Delcorte, J.N. Lambi, Structural characterization and magnetic properties of undoped and copper-doped cobalt ferrite nanoparticles prepared by the octanoate coprecipitation route at very low dopant concentrations. RSC Adv. 8(67), 38621–38630 (2018). https://doi.org/10.1039/c8ra08532c
V.S. Kirankumar, S. Sumathi, Photocatalytic and antibacterial activity of bismuth and copper co-doped cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 29(10), 8738–8746 (2018). https://doi.org/10.1007/s10854-018-8890-x
N.F. Habubi, K.A. Mishjil, S.S. Chiad, Structural properties and refractive index dispersion of cobalt doped SnO2 thin films. Indian J. Phys. 87(3), 235–239 (2012). https://doi.org/10.1007/s12648-012-0223-y
V. Rane, S.T. Ajay, N. Kalarikkal, eds., Microscopy Applied to Materials Sciences and Life Sciences (Routledge, New York, 2018). https://doi.org/10.1201/9781351251587.
K. A. Abd-Elsalam, R. Prasad, eds., Nanobiotechnology Applications in Plant Protection. Nanotechnology in the Life Sciences (Springer, Cham, 2019). https://doi.org/10.1007/978-3-030-13296-5
A.M. Ismail, M.I. Mohammed, S.S. Fouad, Optical and structural properties of polyvinylidene fluoride (PVDF)/reduced graphene oxide (RGO) nanocomposites. J. Mol. Struct. 1170, 51–59 (2018). https://doi.org/10.1016/j.molstruc.2018.05.083
Z. Li, R.-J. Xiao, P. Xu, C. Zhu, S. Sun, D. Zheng, H. Wang et al., Lattice dynamics and contraction of energy bandgap in photoexcited semiconducting boron nitride nanotubes. ACS Nano 13(10), 11623–11631 (2019). https://doi.org/10.1021/acsnano.9b05466
P.R. Jubu, F.K. Yam, V.M. Igba, K.P. Beh, Tauc-Plot scale and extrapolation effect on bandgap estimation from UV–Vis–NIR data—a case study of β-Ga2O3. J. Solid-State Chem. 290, 121576 (2020). https://doi.org/10.1016/j.jssc.2020.121576
B. Rafferty, L.M. Brown, Direct and indirect transitions in the region of the band gap using electron-energy-loss spectroscopy. Phys. Rev. B 58(16), 10326–10337 (1998). https://doi.org/10.1103/physrevb.58.10326
C.M. Soukoulis, Photonic band gap materials. Diffuse Waves Complex Media (1999). https://doi.org/10.1007/978-94-011-4572-5_4
P.I. Devi, K. Ramachandran, J. Exp. Nanosci. 6, 281 (2011)
S. Supriya, S. Kumar, M. Kar, 2017 IEEE 12th Nanotechnology Materials and Devices Conference (NMDC) (2017)
M. El-Masry, R. Ramadan, Appl. Phys. A 128(6), 473 (2022)
A.M. Ismail, M.I. Mohammed, S.S. Fouad, J. Mol. Struct. 1170, 51 (2018)
M. Gaur, D.S. Rana, J. Mater. Sci. Mater. Electron. 26, 1246 (2014)
S. Badawi, S. Alharthi, N.Y. Mostafa, M.G. Althobaiti, T. Altalhi, Appl. Phys. A 125, 1–9 (2019)
M. Ismail, M.H. El-Newehy, M.E. El-Naggar, A. Meera Moydeen, A.A. Menazea, J. Mater. Res. Technol. 9, 11178 (2020)
W.A.A. Mohamed, A. Fahmy, A. Helal, E.A.E. Ahmed, B.A. Elsayed, E.A. Kamoun, E.A.M. Gad, J. Environ. Chem. Eng. 10, 106854 (2022)
M.M. El-Masry, A.S. Ibrahim, J. Mater. Sci. Mater. Electron. 32(13), 18408–18424 (2022)
D.J. Bhagat, G.R. Dhokane, UV–VIS spectroscopic studies of one pot chemically synthesized polyindole/poly(vinyl acetate) composite films. Mater. Lett. 136, 251–253 (2014). https://doi.org/10.1016/j.matlet.2014.08.003
A.A. Kokhanovsky, ed., Light Scattering Reviews 2. Springer Praxis Books (Springer, New York, 2007). https://doi.org/10.1007/978-3-540-68435-0
Y.S. Cho, K.H. Yoon, Dielectric ceramics, in Handbook of Advanced Electronic and Photonic Materials and Devices (Academic Press, San Diego, 2001), pp. 175–199. https://doi.org/10.1016/b978-012513745-4/50040-8.
P. Stoller, V. Jacobsen, V. Sandoghdar, Measurement of the complex dielectric constant of a single gold nanoparticle. Opt. Lett. 31(16), 2474 (2006). https://doi.org/10.1364/ol.31.002474
J.-Y. Kim, T.Y. Kim, J.W. Suk, H. Chou, J.-H. Jang, J.H. Lee, I.N. Kholmanov, D. Akinwande, R.S. Ruoff, Enhanced dielectric performance in polymer composite films with carbon nanotube-reduced graphene oxide hybrid filler. Small 10(16), 3405–3411 (2014). https://doi.org/10.1002/smll.201400363
K. Klyukin, V. Alexandrov, Effect of intrinsic point defects on ferroelectric polarization behavior of SrTiO3. Phys. Rev. B (2017). https://doi.org/10.1103/physrevb.95.035301
M. Hadi, K.M. Batoo, A. Chauhan, O.M. Aldossary, R. Verma, Y. Yang, Tuning of structural, dielectric, and electronic properties of Cu doped Co–Zn ferrite nanoparticles for multilayer inductor chip applications. Magnetochemistry 7(4), 53 (2021). https://doi.org/10.3390/magnetochemistry7040053
H.Y. Zahran, I.S. Yahia, F.H. Alamri, Nanostructured pyronin Y thin films as a new organic semiconductor: linear/nonlinear optics, band gap and dielectric properties. Physica B 513, 95–102 (2017). https://doi.org/10.1016/j.physb.2017.02.026
I.S. Yahia, G.F. Salem, J. Iqbal, F. Yakuphanoglu, Linear and nonlinear optical discussions of nanostructured Zn-doped CdO thin films. Physica B Condens. Matter 511, 54–60 (2017). https://doi.org/10.1016/j.physb.2017.01.030
M. Shkir, V. Ganesh, S. AlFaify, I.S. Yahia, Structural, linear and third order nonlinear optical properties of drop casting deposited high quality nano. J. Mater. Sci. Mater. Electron. 28(14), 10573–10581 (2017). https://doi.org/10.1007/s10854-017-6831-8
M. Tichý, J. Tichá, M. Kočer, Earth Moon Planets 90(1/4), 507–513 (2002). https://doi.org/10.1023/a:1021571425439
I.S. Yahia, H.Y. Zahran, F.H. Alamri, Linear and nonlinear optics of Pyronin Y/flexible polymer substrate for flexible organic technology: new optical approach. Opt. Laser Technol. 95, 124–132 (2017). https://doi.org/10.1016/j.optlastec.2017.03.047
S. Jana, S. Garain, S. Sen, D. Mandal, the influence of hydrogen bonding on the dielectric constant and the piezoelectric energy harvesting performance of hydrated metal salt mediated PVDF films. Phys. Chem. Chem. Phys. 17(26), 17429–17436 (2015). https://doi.org/10.1039/c5cp01820j
B. Mohammadi, A.A. Yousefi, S.M. Bellah, Effect of tensile strain rate and elongation on crystalline structure and piezoelectric properties of PVDF thin films. Polym. Test. 26(1), 42–50 (2007). https://doi.org/10.1016/j.polymertesting.2006.08.003
J. Fu, Y. Hou, M. Zheng, Q. Wei, M. Zhu, H. Yan, Improving dielectric properties of PVDF composites by employing surface modified strong polarized BaTiO3 particles derived by molten salt method. ACS Appl. Mater. Interfaces 7(44), 24480–24491 (2015). https://doi.org/10.1021/acsami.5b05344
H. Liu, J. Zhong, C. Lee, S.-W. Lee, L. Lin, A comprehensive review on piezoelectric energy harvesting technology: materials, mechanisms, and applications. Appl. Phys. Rev. 5(4), 041306 (2018). https://doi.org/10.1063/1.5074184
M. Fortunato, H.C. Bidsorkhi, C.R. Chandraiahgari, G. De Bellis, F. Sarto, M.S. Sarto, PFM characterization of PVDF nanocomposite films with enhanced Piezoelectric Response. IEEE Trans. Nanotechnol. 17(5), 955–961 (2018). https://doi.org/10.1109/tnano.2018.2833201
D. Vasileva, S. Vasilev, A.L. Kholkin, V.Y. Shur, Domain diversity and polarization switching in amino acid β-glycine. Materials 12(8), 1223 (2019). https://doi.org/10.3390/ma12081223
S.K. Mahadeva, J. Berring, K. Walus, B. Stoeber, Effect of poling time and grid voltage on phase transition and piezoelectricity of poly(vinyledene fluoride) thin films using corona poling. J. Phys. D Appl. Phys. 46(28), 285305 (2013). https://doi.org/10.1088/0022-3727/46/28/285305
X. Liu, Xu. Sixing, X. Kuang, D. Tan, X. Wang, Nanoscale investigations on β-phase orientation, piezoelectric response, and polarization direction of electrospun PVDF nanofibers. RSC Adv. 6(110), 109061–109066 (2016). https://doi.org/10.1039/c6ra24473d
D. Damjanovic, Hysteresis in piezoelectric and ferroelectric materials. Sci. Hysteresis (2006). https://doi.org/10.1016/b978-012480874-4/50022-1
S.M. Nakhmanson, M. Buongiorno Nardelli, J. Bernholc, Ab initio studies of polarization and piezoelectricity in vinylidene fluoride and BN-based polymers. Phys. Rev. Lett. (2004). https://doi.org/10.1103/physrevlett.92.115504
D. Chen, Z. Chen, Q. He, J.D. Clarkson, C.R. Serrao, A.K. Yadav, M.E. Nowakowski et al., Interface engineering of domain structures in BiFeO3 thin films. Nano Lett. 17(1), 486–493 (2016). https://doi.org/10.1021/acs.nanolett.6b04512
Acknowledgements
The authors extend their appreciation to the Cairo University and Thebes Academy.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MME: Conceptualization, methodology, formal analysis, data curation, investigation, visualization, writing, review. RR: Methodology, formal analysis, investigation, data curation, writing, review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Masry, M.M., Ramadan, R. Enhancing the properties of PVDF/MFe2O4; (M: Co–Zn and Cu–Zn) nanocomposite for the piezoelectric optronic applications. J Mater Sci: Mater Electron 33, 15946–15963 (2022). https://doi.org/10.1007/s10854-022-08493-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08493-2