Abstract
Sensors for rapid and reliable detection of biomolecules are crucial for clinical medical diagnoses. Here, a rapid, ultra-sensitive, magnetic-assisted biosensor based on resonance Raman scattering at MoS2@Fe3O4 composite nanoflowers is presented. Raman shifts and X-ray photoelectron spectra indicated that the composite was formed via Fe–S covalent bonds. Convenient magnetic separations could be performed because of the superparamagnetic Fe3O4 nanoparticles. MoS2 E12g and A1g Raman peaks were used as probe signals for anti-interference immunoassays. The probe unit of the immunoassay also included goat anti-human IgG molecules that were used as the target analyte. Au substrates coupled with the goat anti-human IgG were used as capture units to form sandwich biosensors. Because of the magnetic enrichment, the detection limit was improved by three orders-of-magnitude and the detection time was reduced from 1.5 h to 1 min. Sandwich biosensors using MoS2@Fe3O4 nanoflowers as Raman probes could be very promising sensors for proteins, antigens, and other immunogenic biopolymers, as well as for corpuscular viruses and cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rapid and accurate biological detection of pathogenic biological materials has been particularly important since the outbreak of COVID-19. Challenges facing most detection methods are complex, time-consuming procedures, and low target concentrations [1]. Biological Raman detection is attractive because of its fingerprint characteristics, narrow half-widths, non-interference from water, and no photobleaching [2]. These offer the possibility of accurate and simple low-concentration bioassays [3]. With regard to shortening detection times, superparamagnetic nanoparticles (NPs) benefit from sensitive responses to external magnetic fields, no coercivity, and no remanence. Superparamagnetic Fe3O4 NPs are biocompatible and can be used in clinical medicine. Its high saturation magnetization enables rapid magnetic enrichment and separations [4, 5]. Fe3O4 NPs can be accurately accumulated at required sites by applying an external magnetic field for rapid enrichment of target molecules. Thus, they improve the detection limit and shorten the detection time [6,7,8].
Magnetic-Raman dual-functional bioassays have demonstrated improved detection limits for miRNA [9], antigens [10], and malachite green isothiocyanate [11]. Moreover, Fe3O4 superparamagnetic nanoparticles have significantly shortened detection times [12], and magnetic separations are more convenient. Previous studies were mainly focused on composites of superparamagnetic nanoparticles and noble metal nanostructures for magnetically assisted surface-enhanced Raman scattering [13, 14].
In contrast to surface-enhanced Raman scattering, resonance Raman scattering (RRS) from inorganic nanomaterials does not require the assistance of metallic nanostructures. The target signals also do not require organic Raman reporters or contrast enhancers, which simplifies the procedure [15,16,17]. Previous reports have used RRS signals from semiconductors (ZnO and ZnS) for femto-molar-level immunological detection [18]. Unlike those for zinc-based semiconductors, the MoS2 bandgap is in the visible light region. Thus, MoS2 RRS can be excited with a 532-nm laser source, instead of the ultraviolet light required for ZnO or ZnS. This makes detection safer, less costly, and no autofluorescence interference has been observed from bulk MoS2 [19,20,21]. Therefore, RRS of MoS2 is promising for biosensing. However, the complexation of Fe3O4 and MoS2 for RRS-based biological detection has not been reported.
MoS2@Fe3O4 nanocomposites were fabricated, and the E12g and A1g vibrational modes of MoS2 were used as RRS probe signals for immunoassays. The magnetic separation and enrichment improved the detection limit and shortened the detection time. This magnetic-assisted rapid RRS detection strategy was simple, low cost, environmentally safe, and could be used for early detection of various pathogens, infectious diseases, and cancers.
2 Experimental section
2.1 Materials
Iron chloride hexahydrate (FeCl3·6H2O, 99.0%), iron sulfate heptahydrate (FeSO4·7H2O, 99.0%), ammonium molybdate tetrahydrate (H24Mo7N6O24·4H2O, 99.9%), N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), and thiourea (CH4N2S, 99%) were purchased from Aladdin (Shanghai, China). Sodium hydroxide (NaOH, Analytical Reagent) was obtained from Sinopharm Chemical Reagent Co. Ltd. Albumin Bovine V (BSA) was obtained from Beijing Gentihold. N-Hydroxysuccinimide (NHS) was purchased from Sigma-Aldrich. Phosphate buffer solution (PBS) was purchased from Meilunbio (Dalian, China). Human IgG and goat anti-human IgG were purchased from Beijing Dingguo Changsheng Biotechnology Co. Ltd. Deionized water (18.2 MΩ·cm) was used throughout.
2.2 Characterization
X-ray diffraction (XRD) patterns were acquired with a Rigaku D/MAX using Cu-Kα radiation (Rigaku Corporation, Japan). The sample morphologies were imaged with scanning electron microscopy (SEM, Quanta FEG-250, FEI Co. Ltd., USA) and transmission electron microscopy (TEM, TecnaiG220S-Twin, USA). X-ray photoelectron spectra (XPS) were acquired with a Thermo Escalate 250Xi XPS spectrometer (Thermo Scientific, USA). RR spectra were acquired with a confocal Raman spectrometer (Horiba Lab Ram HR evolution, Japan). Vibrating sample magnetometry measurements were performed with a 736-VSM Controller (Ohio, USA).
2.3 Synthesis of MoS2@Fe3O4 nanocomposites
MoS2@Fe3O4 nanocomposites were prepared via co-precipitation and hydrothermal methods. Fe3O4 NPs were prepared via co-precipitation with a slight modification [22]. Fe2+ and Fe3+ ions were precipitated with 2-mol/L NaOH until the pH reached 10, and were then mechanically stirred for 30 min under N2. The Fe3O4 NP product was magnetically separated and rinsed with ultrapure water until the supernatant was neutral.
To fabricate MoS2@Fe3O4 nanocomposites, (NH4)6Mo7O24·4H2O and CH4N2S were dissolved in 15 ml of deionized (DI) water in the mass ratio 1:2.2 and sonicated for 15 min. Then, 500 µl of the Fe3O4 NP solution (0.15 mg/ml) was added and the mixture was further sonicated for 30 min, placed into a Teflon container, and kept at 200 °C for 8 h. After allowing it to cool to room temperature, the reaction product was magnetically separated and cleaned with DI water and absolute ethanol, in sequence. The preparation of pure MoS2 was the same, except for the addition Fe3O4 NPs in the hydrothermal process.
2.4 Sandwich Raman immunoassay protocol
The synthesis and working principle of the sandwich biosensor are illustrated in Scheme 1. The biosensor was composed of three parts: a Raman probe signal unit (part A), a Au substrate capture unit (part B), and the immunoassay (part C).
For the preparation of the Raman probe signal unit in part A, a 200-µl solution of MoS2@Fe3O4 composites (0.007 mg/ml) was mixed with the same volume of goat anti-human IgG (660 nM), followed by shaking at 37 °C for 1.5 h. Then, the resulting MoS2@Fe3O4-antibody complex was magnetically enriched and washed twice to remove excess antibodies. Then, 200 µl (0.2 mg/ml) of BSA in a PBS solution was added and the mixture shaken at 37 °C for 1.5 h to block unreacted active sites. Finally, the MoS2@Fe3O4-antibody Raman probe was washed with DI water and then magnetically separated and captured.
The Au substrate capture unit was prepared as described previously[18]. As depicted in part B of Scheme 1, human IgG was used as the target analyte in the immunoassay. In part C of Scheme 1, the goat anti-human-IgG-capture Au substrate was immersed in a human IgG analyte solution with various concentrations. After shaking at 37 °C for 1.5 h, the substrate with the captured analyte was rinsed. Here, two strategies were used to study the effects of the external magnetic field on the immunoassay results. In strategy I, the substrate with the captured analyte was immersed in the solution containing the MoS2@Fe3O4 Raman probe and gently shaken for various periods of time (6 min, 1 h, or 1.5 h) at 37 °C. In strategy II, the substrate was also immersed in the Raman probe solution, but was then placed under an external magnetic field (1.2 T) for several minutes (6 min, 3 min, 1 min, or 0.5 min) instead of being shaken.
3 Results and discussion
3.1 Characteristics of the MoS2@Fe3O4 composites
XRD was performed to determine the crystal structure of the composites (see Fig. 1). The patterns for pure MoS2 and Fe3O4 NPs were also acquired for comparison. The diffraction peaks at 2θ = 29.5°, 34.8°, 42.4°, 52.9°, 56.5°, and 62.1° corresponded to the (220), (311), (400), (422), (511), and (440) planes of Fe3O4 crystals (PDF#72-2303) [23]. The peaks at 2θ = 13.2°, 32.5°, 38.8°, and 58.0° corresponded to the (002), (100), (103), and (110) planes of MoS2 crystals (PDF#37-1492). For the composites, diffraction peaks from both MoS2 and Fe3O4 were observed. Because the composites were magnetically separated and carefully washed, the XRD patterns indicated that the Fe3O4 NPs were combined with MoS2. No unknown peaks appeared, indicating negligible impurities in the MoS2@Fe3O4 composites.
The scanning electron microscope image in Fig. 2a indicated that the MoS2@Fe3O4 composites were 300–900-nm flower-like nanostructures. Magnetic NPs on the petals of MoS2 NFs were marked in the transmission electron microscope image in Fig. 2b. For comparison, scanning electron and transmission electron microscopy images of MoS2 flowers are also shown in Fig. 2c, d and no granular particles were observed.
XPS of MoS2@Fe3O4 NFs and pure Fe3O4 nanoparticles are plotted in Fig. 3. As shown in Fig. 3a, the Fe 2p spectrum via peak fitting had Fe(II)-O (710.2 eV, 724.4 eV) and Fe(III)-O (712.4 eV, 725.6 eV) peaks [24, 25]. In the composite with MoS2, the Fe 2p spectra (Fig. 3b) also indicated divalent and trivalent states. The peaks at 726.4 eV (Fe 2p1/2) and 713.2 eV (Fe 2p3/2) were attributed to Fe(III)-O bonds, and the peaks at 724.1 eV (Fe 2p1/2) and 711.2 eV (Fe 2p3/2) were in agreement with Fe(II)-O bonds [26]. In addition, Fe–S bonds were observed at 710.3 eV [Fe (III)-S] and 708.4 eV [Fe (II)-S] [27], which indicated that the composites were formed with covalent bonds. The peaks at 718.5 eV and 732.5 eV were assigned to Fe (III) and Fe (II) satellites, respectively [28]. The peaks at 530.3 eV and 531.7 eV in the O1s spectra (Fig. 3c) were attributed to Fe-O and O-H in Fe3O4, respectively. The peaks at 530.9 eV in the O1s spectrum (Fig. 3d) corresponded to Mo-O, which may be attributed to oxidization during the synthesis [29, 30]. The characteristic peaks at 228.6 eV and 231.8 eV in the spectra of Mo 3d (Fig. 3e) were attributed to Mo 3d5/2 and Mo 3d3/2, respectively. The component at 225.7 eV corresponded to S 2s in the Mo–S bond [31], and the peak at 235.6 eV corresponded to Mo (VI) [32, 33]. In Fig. 3f, the S 2p1/2 and S 2p3/2 peaks were located at 162.7 eV and 161.5 eV, respectively, in the composite spectra, which were similar to other reported data or MoS2 [34].
Characteristic vibrational fingerprints were reflected in the RRS. As noted above, the 1.29–1.9-eV bandgap of MoS2 enabled resonant 532-nm laser excitation. In Fig. 4a, the Raman peaks at 379.0 cm− 1 and 405.1 cm− 1 were attributed to the in-plane E1 2 g and out-of-plane A1g vibrational modes of MoS2, which were consistent with previous reports [35]. Relative to those for MoS2, both the E1 2 g and A1g modes of MoS2@Fe3O4 NFs shifted to lower energies (377.1 cm− 1 and 403.3 cm− 1, respectively). The shift was attributed to the interaction of MoS2 with Fe3O4 NPs that formed Fe–S bonds observed in the XPS [36]. This changed the stress in the MoS2 lattice, which was affected by its multilayer structure, and eventually led to the change in the lattice vibrational frequency [37].
Figure 4b plots the magnetization curves for Fe3O4 NPs and MoS2@Fe3O4 NFs. Both materials exhibited no significant coercivity or remanence, which verified the superparamagnetic property of the composites. The saturation magnetization of Fe3O4 NPs was approximately 59.3 emu/g, while that of MoS2@Fe3O4 NFs was 24.5 emu/g. Hence, the introduction of the non-magnetic MoS2 NF component decreased the magnetization. Photographs of magnetic enrichment are illustrated in the inset of Fig. 4b. Because the NFs were superparamagnetic, the nanoflowers could be redistributed by slight shaking after the external magnetic field was removed, which enabled rapid biological detection.
3.2 Rapid and ultra-sensitive detection via MoS2@Fe3O4 NF Raman probes
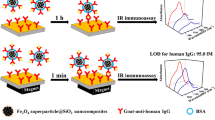
The biological detection of the dual-functional MoS2@Fe3O4 NFs was evaluated utilizing the MoS2 Raman peaks as probe signals and human IgG as the antigen. The detection results using strategy I are shown in Fig. 5a. The RRS intensity decreased with decreasing concentration of the analyte (1 nM, 100 pM, 1 pM, and 100 fM). The weak RRS signal from MoS2 was observed when the analyte concentration was 100 fM, but was almost invisible when the concentration was 10 fM. Therefore, the experimental detection limit using strategy I was 100 fM. The opportunity of coupling between the Raman probe and the Au substrate was enhanced by shaking. However, that was insufficient for detecting very low concentrations. In strategy II, the immunological substrate immersed in the probe solution was placed above a commercial magnet (~ 1.2 T). The “antibody-analyte-antibody” sandwich structure was a bridge between the RRS of MoS2 and the Au substrate. The detection results are shown in Fig. 5b. The RRS signal of the 0.01 fM analyte was negligible, but that for the 0.1 fM analyte was strong. Hence, the detection limit for strategy II was enhanced by three orders-of-magnitude relative to that for strategy I, and was also better than previous reports [38, 39]. Standard deviations of the Raman peak intensities are shown in Fig. 5a, b. To verify the detection specificity, RRS measurements were performed in the absence of the analyte (“blank” in Fig. 5). No MoS2 fingerprint Raman signal was obtained, indicating that nonspecific adsorption did not occur between the Raman probe and the Au substrate.
Another benefit of the magnetic RRS immunoassay was that the detection time was significantly shortened. Because of the magnetic enrichment of the probe, the binding between the capture antibody (goat anti-human IgG on the substrate) and the analyte (human IgG coupled to the probe) was accelerated in a magnetic field during incubation. In strategy I, at least 1.5 h incubation was needed to obtain strong Raman signals (Fig. 5c). However, when a magnet was used to perform the assays (the concentration of the analytes was 100 pM), strong RRS signals were detected after 30 s of incubation, and the signal intensity was stable after 1 min (Fig. 5d). The results thus demonstrated that the Fe3O4 enhanced the detection limit and shortened the detection time.
4 Conclusions
In summary, magnetic MoS2@Fe3O4 NF nanocomposites were fabricated. XPS characterization verified that the composites were covalently bonded. A magnetic-assisted rapid RRS detection method based on MoS2@Fe3O4 NFs shortened detection times from 1.5 h to 1 min, and the detection limit was enhanced by three orders-of-magnitude (from 100 fM to 0.1 fM). The sandwich biosensor exhibited high specificity for human IgG. In addition, the MoS2@Fe3O4 NF-based immunoassay was robust in various pH solutions and chemically stable. The rapid and ultra-sensitive antibody assay should also be applicable to viruses and early diagnoses of diseases such as cancer.
Data Availability
The datasets are available from the corresponding author upon reasonable request.
References
J Li J Wang X Zhang H Chang W Wei 2018 Sensors and Actuators B: Chemical 273 1300 https://doi.org/10.1016/j.snb.2018.07.046
X Wang S-C Huang S Hu S Yan B Ren 2020 Nature Reviews Physics 2 253 https://doi.org/10.1038/s42254-020-0171-y
MK Masud J Na M Younus 2019 Chem Soc Rev 48 5717 https://doi.org/10.1039/c9cs00174c
Y Pang J Shi X Yang C Wang Z Sun R Xiao 2020 Biosens Bioelectron 148 111800 https://doi.org/10.1016/j.bios.2019.111800
X Li J Wei KE Aifantis 2016 J Biomed Mater Res A 104 1285 https://doi.org/10.1002/jbm.a.35654
V Kumar D Kukkar B Hashemi K-H Kim A Deep 2019 Advanced Functional Materials https://doi.org/10.1002/adfm.201807859
K Wu D Su J Liu R Saha JP Wang 2019 Nanotechnology 30 502003 https://doi.org/10.1088/1361-6528/ab4241
S Jamil MRSA Janjua 2017 J. Cluster Sci. 28 2369 https://doi.org/10.1007/s10876-017-1256-3
H Shao H Lin Z Guo 2019 Biosens Bioelectron 143 111616 https://doi.org/10.1016/j.bios.2019.111616
Z Chaloupkova A Balzerova J Barinkova 2018 Anal Chim Acta 997 44 https://doi.org/10.1016/j.aca.2017.10.008
S Kang A Rahman E Boeding PJ Vikesland 2020 Analyst 145 4358 https://doi.org/10.1039/d0an00711k
Y Pang N Wan L Shi 2019 Anal Chim Acta 1077 288 https://doi.org/10.1016/j.aca.2019.05.059
WE Hong IL Hsu SY Huang 2018 J. Mater. Chem. B 6 5689 https://doi.org/10.1039/c8tb00599k
T Zhou M Fan R You 2020 Anal. Chim. Acta 1104 199 https://doi.org/10.1016/j.aca.2020.01.017
Y Li J Heo CK Lim 2015 Biomaterials 53 25 https://doi.org/10.1016/j.biomaterials.2015.02.056
Y Zhou CH Liu Y Sun 2012 J. Biomed. Opt. 17 116021 https://doi.org/10.1117/1.JBO.17.11.116021
M Harz CL Bockmeyer P Rösch RA Claus J Popp 2007 Med. Laser Appl. 22 87 https://doi.org/10.1016/j.mla.2007.06.001
X Hong X Chu P Zou Y Liu G Yang 2010 Biosens Bioelectron 26 918 https://doi.org/10.1016/j.bios.2010.06.066
F Li Y Li J Feng 2018 Biosens Bioelectron 100 512 https://doi.org/10.1016/j.bios.2017.09.048
SS Singha S Mondal TS Bhattacharya 2018 Biosens Bioelectron 119 10 https://doi.org/10.1016/j.bios.2018.07.061
J Jiang Q Shen P Xue 2020 Chem. Select 5 354 https://doi.org/10.1002/slct.201903924
M Tong F Liu Q Dong Z Ma W Liu 2020 J. Hazard Mater 385 121604 https://doi.org/10.1016/j.jhazmat.2019.121604
T Lin J Wang L Guo F Fu 2015 J. Phys. Chem. C 119 13658 https://doi.org/10.1021/acs.jpcc.5b02516
L Wang F Liu A Pal 2021 Carbon 179 327 https://doi.org/10.1016/j.carbon.2021.04.024
H Wu Z Qiu 2021 J. Alloys Comp https://doi.org/10.1016/j.jallcom.2021.160264
X Zhang Y Dong F Pan Z Xiang X Zhu W Lu 2021 Carbon 177 332 https://doi.org/10.1016/j.carbon.2021.02.092
J Su H Hao X Lv X Jin Q Yang 2020 Colloids Surf. A https://doi.org/10.1016/j.colsurfa.2020.124751
Q Hong C Liu Z Wang 2021 Chem. Eng. J. https://doi.org/10.1016/j.cej.2021.129238
N Zhao H Fan M Zhang 2020 Chem. Eng. J. https://doi.org/10.1016/j.cej.2020.124477
C Zhou Q Wang XH Yan 2020 Ceramics Int. 46 15385 https://doi.org/10.1016/j.ceramint.2020.03.083
MA Baker R Gilmore C Lenardi W Gissler 1999 Appl. Surf. Sci. 150 255 https://doi.org/10.1016/S0169-4332(99)00253-6
D Mu Z Chen H Shi N Tan 2018 RSC Adv. 8 36625 https://doi.org/10.1039/c8ra06537c
J Kibsgaard Z Chen BN Reinecke TF Jaramillo 2012 Nat. Mater. 11 963 https://doi.org/10.1038/nmat3439
Z Wang J Zhang T Wen 2020 Sci Total Environ 699 134341 https://doi.org/10.1016/j.scitotenv.2019.134341
HS Matte A Gomathi AK Manna 2010 Angew Chem Int Ed Engl 49 4059 https://doi.org/10.1002/anie.201000009
Z Zhang R Shi F Wang 2020 J. Mater. Sci. 56 5015 https://doi.org/10.1007/s10853-020-05588-1
J Yu W Yin X Zheng 2015 Theranostics 5 931 https://doi.org/10.7150/thno.11802
Y Liu H Wang S Li 2018 Sensors and Actuators B: Chemical 258 402 https://doi.org/10.1016/j.snb.2017.11.083
A Kenry Geldert X Zhang H Zhang CT Lim 2016 ACS Sensors 1 1315 https://doi.org/10.1021/acssensors.6b00449
Acknowledgements
This work was supported by the “111” Project of China (D17017), the Developing Project of Science and Technology of Jilin Province (202002040JC, 20200201266JC, 20200201271JC), the International Science and Technology Cooperation Project of Jilin province (20190701029GH) and the Project of Education Department of Jilin Province (JJKH20200730KJ). We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
TZ contributed toward writing original draft, investigation, data curation, and formal analysis. XC contributed toward conceptualization, manuscript writing, reviewing, and editing, methodology, visualization, project administration, and funding acquisition. FJ contributed toward investigation, manuscript writing, reviewing, and editing, funding acquisition. MX contributed toward manuscript writing, reviewing, and editing, and funding acquisition. YZ collected resources, and contributed toward manuscript writing, reviewing, and editing, and funding acquisition. JL contributed toward conceptualization, supervision, manuscript writing, reviewing, and editing, and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Ethical approval
The authors declare that all data in this paper are original and have not been published in any other journal. This paper did not involve unethical treatment of animals.
Consent for participate
All authors have provided consent to participate.
Consent for publication
All authors have read the manuscript provided for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, T., Chu, X., Jin, F. et al. Superparamagnetic MoS2@Fe3O4 nanoflowers for rapid resonance-Raman scattering biodetection. J Mater Sci: Mater Electron 33, 15754–15762 (2022). https://doi.org/10.1007/s10854-022-08477-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08477-2