Abstract
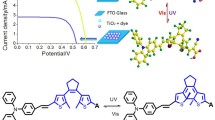
Novel dyes F1-3 based on bichalcophene-pyrimidine-2,4,6-trione derivatives with dual anchoring were developed, synthesized, and evaluated as sensitizers and co-sensitizers for dye-sensitized solar cells (DSSCs). F1 displayed the best DSSC performance (η, PCE = 4.41%) and the highest photovoltaic parameters, which were as follows: current density, JSC = 10.66 mA cm−2, photovoltage, VOC = 0.654 V, and fill factor, FF = 64.3%. These findings can be ascribed to F1’s superior optical and electrochemical characteristics when compared to other structures such as F2 and F3. Interestingly, devices that rely on the F1 + N-719 co-sensitization approach had greater photocurrent and photovoltage than the standard N-719 dye, generating a power conversion efficiency (PCE) of 9.97%. This improved performance was mostly due to a higher JSC value of 23.28 mA cm−2 for the dye F1 and a maximum molar extinction coefficient in the 350–550 nm region, which enhanced the light-harvesting capacity of the N-719 dye.













Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
B. Oregan, M. Gratzel, A low-cost high-efficiency solar cell based on dye sensitized colloidal TiO2 films. Nature 353, 737–740 (1991)
M. Gratzel, Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 44, 6841–6851 (2005)
A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, H. Pettersson, Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010)
X. Meng, C. Yu, X. Zhang, L. Huang, M. Rager, J. Hong, J. Qiu, Z. Lin, Active site senriched carbon matrix enables efficient triiodide reduction in dye-sensitized solar cells: an understanding of the active centers. Nano Energy 54, 138–147 (2018)
X. Meng, C. Yu, X. Song, J. Iocozzia, J. Hong, M. Rager, H. Jing, S. Wang, L. Huang, J. Qiu, Z. Lin, Scrutinizing defects and defect density of selenium-doped Graphene for high-efficiency triiodide reduction in dye-sensitized solar cells. Angew. Chem. Int. Ed. 130, 4772–4776 (2018)
M. Barrera, I. Crivelli, B. Loeb, On the performance of ruthenium dyes in dye sensitized solar cells: a free cluster approach based on theoretical indexes. J. Mol. Model. 22, 118 (2016)
S. Aghazada, M.K. Nazeeruddin, Ruthenium complexes as sensitizers in dye sensitized solar cells. Inorganics 6, 52 (2018)
C. Woodward, T. Rüther, C. Coghlan, T. Jones, Y. Hebting, R. Cordiner, R. Dawson, D. Robinson, C. Forsyth, G. Wilson, Preparation of tetracarboxylated bis-bipyridine Ruthenium dyes: synthesis, structural and electronic characterization. Chem. Plus Chem. 83, 691–703 (2018)
A. Sen, A. Gro, β effect of electron withdrawing/donating groups on the sensitizing action of the novel organic dye “3-(5-(4-(Diphenylamino)Styryl)Thiophen-2-Yl)-2-Cyanoacrylic Acid” for N-Type dye sensitized solar cells: a theoretical study. J. Phys. Chem. C 124(16), 8526–8540 (2020)
A. Mishra, M.K.R. Fischer, P. Büuerle, Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48(14), 2474–2499 (2009)
S. Singh, M. Chandrasekharam, K. Gupta, A. Islam, L. Han, G. Sharma, Co-sensitization of amphiphilic ruthenium(II) sensitizer with a metal free organic dye: improved photovoltaic performance of dye sensitized solar cells. Org. Electron. 14, 1237–1241 (2013)
J. Luo, Z. Wan, C. Jia, Y. Wang, X. Wu, A co-sensitized approach to efficiently fill the absorption valley, avoid dye aggregation and reduce the charge recombination. Electrochim. Acta 215, 506–514 (2016)
Y.-J. Chen, Y.-C. Chang, L.-Y. Lin, W.-C. Chang, S.-M. Chang, Enhancing the spectral response of mesoporous ZnO films of dye–sensitized solar cells by incorporating metal-free organic sensitizer and N719 dye. Electrochim. Acta 178, 414–419 (2015)
A. Mishra, M. Fischer, P. Bäuerle, Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48, 2474–2499 (2009)
Z. Ning, Y. Fu, H. Tian, Improvement of dye-sensitized solar cells: what we know and what we need to know. Energy Environ. Sci. 3, 1170–1181 (2010)
M. Al-Eid, S. Limb, K. Park, B. Fitzpatrick, C. Han, K. Kwak, J. Hong, G. Cooke, Facile synthesis of metal-free organic dyes featuring a thienylethynyl spacer for dye sensitized solar cells. Dyes Pigm. 104, 197–203 (2014)
R.Y.-Y. Lin, F.-L. Wu, C.-H. Chang, H.-H. Chou, T.-M. Chuang, T.-C. Chu et al., Y-shaped metal-free D–π–(A)2 sensitizers for high-performance dye-sensitized solar cells. J. Mater. Chem. A 2, 3092–3101 (2014)
S. Fernandes, M. Castro, D. Ivanou, A. Mendes, M. Raposo, Push-pull heterocyclic dyes based on pyrrole and thiophene: synthesis and evaluation of their optical, redox photovoltaic properties. Coatings 12(1), 34 (2022)
K. Kavya, P. Naik, A. Adhikari, Simple thiophene based organic dyes as active photosensitizers for DSSC application: from molecular design to structure property relationship. J. Nano-Electron. Phys. 12, 02039 (2020)
S. Rouhani, M. Hosseinnezhad, N. Sohrab, K. Gharanjig, A. Salem, Z. Ranjbar, Investigation of the effect of rGO/TiO2 on photovoltaic performance of DSSCs devices. Prog. Color. Color. Coat 15, 123–131 (2022)
L. Zhang, J. Cole, Anchoring groups for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 7, 3427–3455 (2015)
F. Ambrosio, N. Martsinovich, A. Troisi, Effect of the anchoring group on electron injection: theoretical study of phosphonated dyes for dye-sensitized solar cells. J. Phys. Chem. C 116, 2622–2629 (2012)
B. Hosseinzadeh, A.S. Beni, A.N. Chermahini, R. Ghahary, A. Teimouri, Novel organic dyes with anchoring group of barbituric/thiobarbituric acid and their application in dye-sensitized solar cells. Synth. Met 209, 1–10 (2015)
S. Badawy, R. Su, A. Fadda, E. Abdel-Latif, A. El-Shafei, M. Elmorsy, Highly efficient (N-benzothiazolyl)-cyanoacetamide based co-sensitizers for high efficiency dye-sensitized solar cells. Optik 249, 168274 (2022)
K. Harmanda, K. Tavera, M. Gezgin, M. Nebioğlu, İ Şişman, G. Jirón, D. Atilla, A. Gürek, A new sterically hindered asymmetric zinc phthalocyanine as an efficient sensitizer for dye-sensitized solar cells. New J. Chem. 46, 714–725 (2022)
M. Elmorsy, E. Abdel-Latif, S. Badawy, A. Fadda, New cyanoacetanilides based dyes as effective co-sensitizers for DSSCs sensitized with ruthenium (II) complex (HD-2). J. Mater. Sci. Mater. Electron. 31, 7981–7990 (2020)
L. da Silva, M. Sánchez, M. Rodriguez, H. Freeman, Isomeric tetrazole-based organic dyes for dye-sensitized solar cells: structure-property relationships. J. Mol. Struct. 1250, 131749 (2022)
M. Eltoukhi, A. Fadda, E. Abdel-Latif, M. Elmorsy, Low cost carbazole-based organic dye bearing the acrylamides and 2-pyridone moieties for efficient dye-sensitized solar cells. J. Photochem. Photobiol. A 426, 113760 (2022)
S. Fernandes, M. Castro, L. Mesquita, L. Andrade, A. Mendes, M. Raposo, Synthesis and characterization of novel thieno [3, 2-b] thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells. Dyes Pigm. 136, 46–53 (2017)
D. Babu, R. Su, A. El-Shafei, A. Adhikari, From molecular design to cosensitization; High performance indole based photosensitizers for dye-sensitized solar cells. Electrochim. Acta 198, 10–21 (2016)
M. Nazeeruddin, M. Zakeeruddin, R. Humphry-Baker, M. Jirousek, P. Liska, N. Vlachopoulos, M. Grätzel, Acid−Base equilibria of (2, 2 ‘-Bipyridyl-4, 4 ‘-dicarboxylic acid) ruthenium (II) complexes and the effect of protonation on charge-transfer sensitization of nanocrystalline titania. Inorg. Chem. 38, 6298–6305 (1999)
Y. Shu, A. Mikosch, K.N. Winzenberg, P. Kemppinen, C.D. Easton, A. Bilic, C.M. Forsyth, C.J. Dunn, T.B. Singha, G.E. Collis, N-Alkyl functionalized barbituric and thiobarbituric acid bithiophene derivatives for vacuum deposited n-channel OFETs. J. Mater. Chem. C 2(20), 3895–3899 (2014)
M.A. Ismail, M.H. Abdel-Rhman, G.A. Abdelwahab, W.S. Hamama, H.M. El-Shafeai, W.M. El-Sayed, Synthesis of new thienylpicolinamidine derivatives and possible mechanisms of antiproliferative activity. RSC Adv. 10, 41165–41176 (2020)
M.S. McClure, F. Roschangar et al., A practical one-pot synthesis of 5-aryl-2-furaldehydes. Synthesis 11, 1681–1685 (2001)
M.A. Ismail, D.W. Boykin, C.E. Stephens, An efficient synthesis of 5,5’-diaryl-2,2’-bichalcophenes. Tetrahedron Lett. 47, 795–797 (2006)
A. Mishra, C. Ma, P. Bäuerle, Functional oligothiophenes: molecular design for multidimensional nanoarchitectures and their applications. Chem. Rev. 109, 1141–1276 (2009)
Z. Iqbal, W.-Q. Wu, D.-B. Kuang, L. Wang, H. Meier, D. Cao, Phenothiazine-based dyes with bilateral extension of π-conjugation for efficient dye-sensitized solar cells. Dyes Pigm. 96, 722–731 (2013)
J. Yang, P. Ganesan, J. Teuscher, T. Moehl, Y. Kim, C. Yi, P. Comte, K. Pei, T. Holcombe, M. Nazeeruddin, J. Hua, S. Zakeeruddin, H. Tian, M. Grätzel, Influence of the donor size in D-π-A organic dyes for dye-sensitized solar cells. J. Am. Chem. Soc. 136, 5722–5730 (2014)
J. Luo, Z. Wan, C. Jia, Y. Wang, X. Wu, X. Yao, Co-sensitization of dithiafulvenyl-phenothiazine based organic dyes with N719 for efficient dye-sensitized solar cells. Electrochim. Acta 211, 364–374 (2016)
H. Tian, X. Yang, J. Cong, R. Chen, C. Teng, J. Liu, Y. Hao, L. Wang, L. Sun, Effect of different electron donating groups on the performance of dye-sensitized solar cells. Dyes Pigm. 84, 62–68 (2010)
Z.-S. Huang, H.-L. Feng, X.-F. Zang, Z. Iqbal, H. Zeng, D.-B. Kuang, L. Wang, H. Meier, D. Cao, Dithienopyrrolobenzothiadiazole-based organic dyes for efficient dye-sensitized solar cells. J. Mater. Chem. A 2, 15365–15376 (2014)
J. Zhao, X. Yang, M. Cheng, S. Li, L. Sun, Molecular design and performance of hydroxylpyridium sensitizers for dye-sensitized solar cells. ACS. Appl. Mater. Interfaces 5, 5227–5231 (2013)
M. Frisch, G.W. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, Eur. J. Inorg. Chem. 3690–3697 (2017), www.eurjic.org 3697 © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, W. C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009.
J.N. Clifford, E. Martínez-Ferrero, A. Viterisi, E. Palomares, Sensitizer molecular structure-device efficiency relationship in dye sensitized solar cells. Chem. Soc. Rev. 40, 1635–1646 (2011)
M. Rashid, D. Hayati, K. Kwak, J. Hong, Theoretical investigation of azobenzene-based photochromic dyes for dye-sensitized solar cells. Nanomaterials 10, 914 (2020)
Y.-J. Chen, Y.-C. Chang, L.-Y. Lin, W.-C. Chang, S.-M. Chang, Enhancing the spectral response of mesoporous ZnO films of dye-sensitized solar cells by incorporating metal-free organic sensitizer and N719 dye. Electrochim. Acta 178, 414–419 (2015)
N. Neale, N. Kopidakis, J. van de Lagemaat, M. Grätzel, A. Frank, Effect of a coadsorbent on the performance of dye-sensitized TiO2 solar cells: shielding versus band-edge movement. J. Phys. Chem. B 109, 23183–23189 (2005)
M.R. Elmorsy, L. Lyu, E. Abdel-Latif, S. Badawy, A.M. El-Shafei, A. Fadda, Co-sensitization of HD-2 complex with low-cost cyanoacetanilides for highly efficient DSSCs. Photochem. Photobiol. Sci. 19, 281–288 (2020)
P. Naik, R. Su, M.R. Elmorsy, A. El-Shafei, A.V. Adhikari, Investigation of new carbazole based metal-free dyes as active photosensitizers/co-sensitizers for DSSCs. Dyes Pigm. 149, 177–187 (2017)
G. Oskam, B.V. Bergeron, G.J. Meyer, P.C. Searson, pseudohalogens for dye-sensitized TiO2 photoelectrochemical cells. J. Phys. Chem. B 105, 6867–6873 (2001)
Y. Hua, B. Jin, H. Wang, X. Zhu, W. Wu, M.-S. Cheung, Z. Lin, W.-Y. Wong, W.-K. Wong, Bulky dendritic triarylamine-based organic dyes for efficient co-adsorbent-free dye-sensitized solar cells. J. Power Sources 237, 195–203 (2013)
Funding
The authors received no funding for this research.
Author information
Authors and Affiliations
Contributions
FHA: synthesis, methodology, and graphical plots. MAI and EA-L: supervision, initial corrections, and comments. AAA: optical proprieties measurements, data analysis and revision. MRE: writing original draft, data analysis, editing, proofreading, and manuscript handling. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdelhamed, F.H., Ismail, M.A., Abdel-Latif, E. et al. Design and synthesis of novel bichalcophene derivatives with double anchoring groups for dye-sensitized solar cell applications: sensitization and co-sensitization with N-719. J Mater Sci: Mater Electron 33, 15665–15678 (2022). https://doi.org/10.1007/s10854-022-08470-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08470-9