Abstract
Multiferroic (x)BaTiO3/(1 − x)Li0.5Fe2.5O4 (0.0 ≤ x ≤ 1) perovskite/spinel nanocomposite was synthesized using a modified citrate auto-combustion method. Both BaTiO3 (BTO) and Li0.5Fe2.5O4 (LFO) phases along with their composites were successfully characterized using X-ray diffraction, Fourier transformation infrared spectroscopy, and X-ray absorption fine structure spectroscopy. The nanoscale, morphology, and magnetic properties were imaged and acquired using high resolution transmission electron microscopy, atomic force microscopy, and vibrating sample magnetometer. Synchrotron radiation-based elemental selective XAFS technique was performed around Fe K-edge to obtain the selective and the detailed local structural information of Li0.5Fe2.5O4 nanoparticles (NPs). The main remarkable result in this work is the enhancement in the coercivity (HC) of LFO NPs after compositing with BTO particularly at x = 0.6, and at x = 0.8. Based on the collected results, BTO/LFO nanocomposite can be seen as a good candidate for different technological applications including magnetically modulated piezoelectric, safety rechargeable batteries, and multilayer ceramic capacitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tailoring and controlling the composite materials properties are the key in the advanced modern and future technology that meets the human needs and ambitios especially in solving the energy and environment crises [1]. Composite is a tailored controlled material system which consists of two or more distinguished separate phases [2]. The aim of the nanocomposite fabrication is to get a new advanced material system with novel characteristics and highly efficient performance. Accordingly, the main advantage of composites is the ability to tailor new materials with new properties for modern applications. Basically, the output new composite properties depend on the different own physicals and chemicals features of the original constituent phases [3]. Furthermore, nanocomposite is a subset of composites that takes the advantage of the unique materials properties on the small scale (at which at least one of its constituents is in nanoscale) [4]. Composites usually have three parts; namely, a matrix, structural constituents and an the hetero-interface [5]. The matrix is a homogeneous phase which surrounds the other phases (fillers), and the hetero-interface which is the boundary area among the different phases and the matrix network. With the development of electronic technology, composite materials have been widely used for electronic devices where higher densities, limited space and multifunction are required [6]. On the other hand, multiferroic nanocomposite is an important class of nanocomposite that have become in attention because of their promising applications such as in energy conversion, fuel cells, storage, shape memory actuation and solid-state refrigeration, next-generation spintronics devices or multiple state memory elements, and transducers with magnetically modulated piezoelectricity [1,2,3,4,5,6,7]. In particular, biferroic is a class of multiferroics that shows the simultaneous ferroelectric and ferromagnetic order parameters [8]. Generally, the concept of multiferroic stands for the presence of an interaction between the ferroelectric and ferromagnetic subsystems. That is, the external electric field affects the magnetic properties, and vice versa; the magnetic field affects the electric polarization [3]. It was reported that, it is not easy to design a single-phase material that shows the coexisting of both ferroelectric and ferromagnetic ordering at room temperature [3]. Therefore, the ferroelectric/ferrite composite is considered as a good solution for the material system that gives the multiferroic (magnetoelectric) properties [1,2,3,4,5]. Lithium ferrite is an inverse spinel crystal structure, where three fifths of the Fe3+ cations (1.5) and Li1+ cations are supposed to be octahedrally coordinated in B site (octahedral) sublattice. Lithium ferrite is a good candidate in numerous technological applications such as in safety rechargeable batteries, because it possesses high resistivity, low dielectric loss at high frequencies and high Curie point [9]. The second phase in the composite (Barium titanate) is reported as a very important ferroelectric material due to its echo-friendly ferroelectric character; free from toxics for the environment [10]. Barium titanate (BTO) is a perovskite type oxide group which is the most popular ferroelectric material used in multi-layer ceramic capacitors, piezoelectric and positive temperature coefficient devices [10]. The ferroelectric behavior of BTO is arising from the crystal asymmetry which is associated with the displacement of Ti ion from its body-centered positions in the crystal lattice [11, 12]. Zheng et al. reported that BTO NPs can enhance the multifunctional properties of Li0.5Fe2.5O4 [8].
One important point here to be mentioned is that, this work is a completion of our previously published work by Imam et al. [2]; that focused on the systematic study of the dielectric properties of BTO/LFO nanocomposite. The (x)BTO/(1 − x)LFO composites have been synthesized by a modified citrate precursor auto combustion method and characterized using XRD and Near Edge XAFS (XANES) to investigate the average and electronic structural. The Synchrotron based XAFS technique is an important technique for probing the electronic/local structure of the material, even for more diluted and disordered nanomaterials and multicomponent materials such as nanocomposites [13,14,15].
In this work, the XAFS technique was utilized in order to probe the own atomic/local structure of Lithium ferrite at Fe K-edge before incorporating it in the target LFO/BTO nanocomposite. The XAFS spectrum consists of two portions namely, XANES or X-ray absorption near edge structure spectroscopy and EXAFS or Extended X-ray absorption fine structure spectroscopy [14]. Generally, XANES gives detailed information about the electronic and oxidation states of the absorbing ions and provides details about the site symmetry and local coordination chemistry around the absorber [2, 15]. While EXAFS is a brilliant synchrotron technique mapping the fine atomic environment surrounding the absorber ion in the media. Moreover, EXAFS images the induced local distortions around the target selective ion upon doping with impurities. Furthermore, bond length R, mean-square relative displacement σ2, and coordination numbers N of the coordination shells around the selective target absorbing ion are the main output structural parameters from the EXAFS fitting [16]. The local/atomic structure in addition the cation site occupancy influence strongly on the magnetic properties. From the EXAFS fitted bond distance, the cation distribution between the sublattices of the spinel structure can be identified [13,14,15,16].
In this contribution, we intended to study the magnetic properties of the target composite and linked the observed magnetic behavior with the own fine structure of the magnetic phase and the weight percent of the BTO phase. Consequently, the main aim of this work is to focus on the optimization of the magnetic properties of BaTiO3/Li0.5Fe2.5O4 multiferroics prepared via modified relatively low-temperature combustion technique. The prepared (x)BaTiO3/(1 − x)Li0.5Fe2.5O4 nanocomposites (x = 0, 0.2, 0.4, 0.6, 0.8 and 1) were characterized using various experimental techniques such as FTIR, HRTEM, AFM, and SR-XAFS. The main originality of this work lies in exploring the role of the chemical, fine/electronic, and imaging properties on the magnetic performance of (x)BaTiO3/(1 − x)Li0.5Fe2.5O4 nanocomposites.
2 Experimental procedures: materials and methods
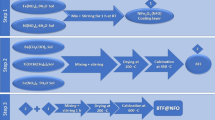
Nanocomposite with the nominal composition of (x)BTO/(1 − x)LFO with x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 were synthesized by the modified citrate precursor auto combustion method. The details of the synthesis process of BTO and LFO were described previously in the flowchart mapped in our published paper by Imam et al. [2]. While Fig. 1 shows the coupling between (x)BaTiO3 and (1 − x)Li0.5Fe2.5O4 at x = 0.0, 0.2, 0.4. 0.8, and 1.0. In addition, the crystal structure of the multiferroic composite and their constituent phases were determined by X-ray diffractometer [Proker D8 model with CuKα radiation (λ = 1.5418 Å)] in a wide range of Bragg’s angle (2θ) ranging from 10° to 70° at room temperature. The XRD results were published in our paper [2]. Herein we recall the XRD results to confirm the formation of the nanocomposite without any extra phases. Also, the XRD results were combined with those of the local structure which were extracted from the XAFS spectra in order the explain the magnetic behavior of LFO in the target composite.
Transmission electron microscope (HRTEM, JEOL-1010) was used to image the morphology (particle shape and size) of the samples. The infrared spectra (FTIR) of the investigated samples recorded (IR spectrometer model FT/IR-6100) in the range from 4000 to 400 cm−1 using KBr matrix. FTIR was used to provide information about the chemical/bond nature of the coupling between LFO and BTO nanoparticles. The atomic force microscopy (AFM Agilent 5500) is used for characterizing nanocomposites of (x)BaTiO3 and (1 − x)Li0.5Fe2.5O4 at x = 0.0, 0.2, 0.4. 0.8, and 1.0. It determines size, morphology, surface texture and roughness [17,18,19].
The measurement of the magnetic susceptibility was performed using Faraday's method [20] at different temperatures ranging from 300 to 1023 K as a function of different applied magnetic field intensities of (1010, 1340, and 1660 Oe). The temperature of the samples was measured using K-type thermo-couple with a junction very closed to the sample keeping it free. The accuracy of magnetic susceptibility measurements was ± 1%.
The magnetic parameters were extracted from the analysis of the collected M−H hysteresis loops. Vibrating sample magnetometer (VSM; Lake Shore -7410-USA) with a maximum applied magnetic field of ± 20 kOe was employed [20].
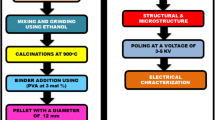
The Italian 11.1 XAFS beamline of Elettra synchrotron radiation source in Trieste-Italy was used for XAFS data collection around 7112 keV of Fe K-edge [21]. The sample was finely powdered and composited into polyvinyl pyrrolidine (PVP) matrix and then pressed into pellet with a diameter of 13 mm and with a proper highly uniform optical thickness. XAFS data collection of both Li0.5Fe2.5O4/PVP sample and the reference of iron foil were simultaneously measured in the transmission mode and at room temperature (295 K). Four scans were acquired, merging, and averaging to assure the spectral reproducibility. The extended XAFS (EXAFS) data was reduced using the Demeter-0.9.26 package standard procedures (version 0.9.26, Naval Research Laboratory, Washington, DC, 20375, USA) [22, 23]. Fits of the k2 weighted EXAFS data were carried out in R space using theoretical functions from the FEFF9 code. High-quality k-space data were obtained up to 12 Å−1 and the full fit range was 1.00–3.7 Å (R-space). The refined input fitting EXAFS parameters were the amplitude reduction factor S02, mean-square relative displacement σ2, the interatomic bond lengths R, the absorption edge energy shift between the sample and the theoretical model ΔE0. The refinement of the relative contribution of tetrahedral and octahedral site-occupancy for the Fe ions was also included in the EXAFS fitting analysis [13].
3 Results and discussion
3.1 Average crystal, chemical, and morphological structural analyses
The detailed study of the average crystal structure of for (x)BTO/(1 − x) LFO nanocomposite was essentially described in our previously published paper [2]. The XRD patterns confirmed the formation of (x)BTO/(1 − x)LFO nanocomposites with the two discrete original constituent phases without the appearance of any secondary/impurity extra phases. The average crystallite size of (x)BTO/(1 − x) LFO composite was around 55 ± 5 nm.
Figure 2 shows the acquired FTIR spectra of BTO/LFO nanocomposites in the absorption mode. The main absorption FTIR peak positions are tabulated in Table 1. The functional groups corresponding to the FTIR spectra peak of the two composite phases are marked as shown in Fig. 2. The apparent FTIR absorption peaks support the XRD results, described in [2], and confirm the successful formation of the only two distinguished separate phases with a high crystalline quality and free of secondary phases.
Figure 3 illustrated the typical HRTEM morphology. The micrographs indicated the nanostructure nature with the spherical like shape at x = 0. While the HRTEM image exhibits rod-like morphology for BTO (x = 1.0) with a length of 120 nm and a diameter of 43 nm. HRTEM images prove that the modified citrate auto-combustion method was a successful and facile method for obtaining nanorod-like shape of the perovskite material (BTO). The average crystallite size of the BTO NPs obtained from X-ray diffraction was around 47 nm [2]. The selected area electron diffraction (SAED) pattern of the sample with x = 0.2 is shown in the inset of Fig. 3. The bright, sharp, and ordered rings show the polycrystalline nature of the sample, and each ring refers to a specific crystal plane of the polycrystalline material. The existence of the two different spots at each ring represents the diffraction from the two different constituent phases within the composite. The reciprocal of the distance between the two successive rings gives the value of the d spacing.
It is known that the AFM topographic image of the sample is obtained by plotting the deflection of the cantilever versus its position on the sample surface. Alternatively, it is possible to plot the height position of the translation stage. This height is controlled by a feedback loop, which maintains a constant force between the tip and the sample.
Figure 4a, b shows the surface AFM topography and the average roughness (Ra) of the BaTiO3 sample of (Ra ≃ 29.3 nm). The contrast in the AFM image arises from the dependence of the force between the tip and the sample on both tip–sample separation and the own properties of both the tip and the sample [24]. The graph shows that the grain size of BaTiO3 is ≃ 59.1 nm, confirming the polycrystalline structure in the BaTiO3 grains.
3.2 Local/atomic structural study: XAFS analysis
The Fe K-edge XAFS technique was accessed to describe the local geometry and electronic structure of/around Fe absorbing atom in the LFO phase. The Fe K-edge XANES probes the unoccupied 4p orbitals with 1s → 4p dipole transitions and unoccupied 3d orbitals via the much weaker intensity pre-edge, 1s → 3d, transitions. While the Fe K-edge EXAFS quantifies the interference effects due to electron scattering from the surrounding ions.
The local/atomic structure is necessary for explaining and understanding the magnetic behavior of Li0.5Fe2.5O4 NPs. Accordingly, EXAFS study is a useful technique for imaging the fine local structure in a short-range order surrounding the absorbing ions (up to 5 Å). The normalized Fe K-edge absorption full XAFS spectrum (μ(E)) obtained for Li0.5Fe2.5O4 NPs is plotted and presented in Fig. 5. As aforementioned above, the XAFS data was collected at room temperature (295 K) in the transmission geometry. The XAFS spectrum shows three different energy regions with respect to the main maximum peak or the white-line position. These three regions include the pre-edge energy region that is before the main absorption edge (around 7114.44 ± 0.04 eV). Second, the main absorption edge which could be selected differently. The red circle in the normalized XAFS spectrum represents the main absorption edge opted position in the half-hight of the edge step. The pre-edge plus the main absorption edge, in addition to the white-line (maximum intensity peak) are described as the near edge XAFS (XANES) region or the X-ray absorption near edge spectroscopy which is a fingerprint characteristic feature (the inset of Fig. 5). The post-edge secondary peaks appeared a few 10 eV above the main absorption edge are attributed to the multiple scattering from the surrounding back scatterers shells (Fe, Li, or/and oxygen ions). After the post edge, the oscillatory signal region is the extended X-ray absorption fine structure spectroscopy (EXAFS). These oscillations are expressed by an EXAFS equation reported in [25].
The oxidation state/s of Fe was/were identified from the first derivative of the XANES spectra (dμ(E)/dE) and by comparing the peak positions and intensities of the sample spectrum along with those of the iron reference model compounds as shown in Fig. 6. The first derivative of the XANES spectrum gives a more resolved and distinguished peak position more than that in case of original XANES direct spectrum [13]. It is observed that the first inflection point corresponding to the pre-edge position of the LFO sample is very close to that of the Fe2O3 (Fe3+) reference compound, confirming the trivalent character of iron ions within the LFO NPs. Also, the second or the highest intensive inflection point corresponding to the main absorption edge or the maximum of the white-line position of LFO is almost coincident with that of Fe2O3 (Fe3+) spectrum, supporting the same result of the formation of Fe ions in the Fe3+ oxidation.
On the other hand, the extraction of the normalized EXAFS part of the XAFS spectrum multiplied by κ2 is revealed in Fig. 7a. The well seen magnified EXAFS oscillations was taken up to k ≃ 12 Å−1. FTs or Fourier transform amplitude of the k2 weighted EXAFS signal is plotted in Fig. 7b, while the FTs envelope of the EXAFS oscillations is plotted in Fig. 7c. The variation in the amplitude of the FTs envelope indicates the different surround environments (back scatterers ions) around the absorbing ion. The FTs amplitude spectrum shows that the average Fe–O bond distance in the first coordination nearest neighbors is located around 1.477 Å−1. The second peak represents the Fe–Fe/Li bond lengths in the second coordination shell. It is clear that the second peak is splitting into two different distinguished peaks representing the FeB–FeB and FeB–FeA/FeA–FeA, respectively. This splitting confirms the distribution of Fe absorbing ions between the two tetrahedral and octahedral sublattices of the spinel structure shown in Fig. 7. The ratio between the FTs amplitude of the octahedral peak to that of the tetrahedral peak could be used to estimate the cation distribution. Accordingly, it was found that the calculated cation distribution was as follow:
a k2χ(k) EXAFS oscillations. b Fourier transform (FT) magnitude corresponding to k2χ(k) EXAFS signal, and c Envelope of EXAFS signal of real and imaginary parts in q-space. Schematic structure of the spinel ferrite [26]
where, ϒ is the vacancy, representing the structure disorder that saturates the deficiency of Fe ions in tetrahedral site. The detected structure disorder from EXAFS analysis will affect the magnetic behavior as will be shown in the discussion of the magnetic properties.
Furthermore, and for determining the fine local structural parameters such as the near neighbor bond lengths of the Fe ions; the EXAFS spectrum was fitted using the theoretical structural model of Li0.5Fe2.5O4 NPs (ICDD # 88-0671) [27]. Figure 8 explores the magnitude of FTs of EXAFS signal in k-range from 3 to 12 Å, across Fe K-edge along with the respective best fit of Li0.5 Fe2.5O4 NPs. On the other words, Fig. 8 shows the comparison between the best-fit results and the collected EXAFS spectrum reported for the FTs magnitude and the imaginary part (Left Panel), and the extracted k3 signal (Right Panel).
Table 2 listed the well-fit structural parameters of the nearest and next nearest coordination shells of Li0.5Fe2.5O4 NPs. The R-Factor of the fit was in the value ≃ 0.02 and the overall reduction factor S02 was fixed to be 1.00 for all the selected scattering paths and all coordination neighbors in order to reduce the correlations among the fitting parameters. Whereas only the single scattering paths (SSP) were considered during the EXAFS fit. The first peak around 2 Å is corresponding to the two Fe–O bond lengths, which involves FeA–O (1.948 Å) and FeB–O (2.121 Å), as a reflection from the tetrahedral and octahedral site occupancy, respectively. The second splitting peak in the range of 2.5–4 Å is arisen from the different possible contributions from the FeB–FeB (3.024 Å)/FeB–LiB (3.143 Å), FeA–FeA (3.486 Å), and FeA–FeB (3.546 Å) bonds. It is observed that the Mean Square Relative Displacement (σ2) increases from 0.007 Å2 (for the first coordination shell) up to 0.040 Å2 (for the higher coordination shells). This increase is expected due to the larger disorder of the superficial shells.
The fitted inversion parameter (x), (representing the percentage % of the occupancy of the tetrahedral (A) and octahedral (B) sites by Fe3+ions), has the value around 0.88. Consequently, the extracted cation site distribution is in this form of:
Where, ϒ is the vacancy representing the structure disorder that saturates the deficiency of Fe in the tetrahedral site, as mentioned above.
It is remarkable that the calculated and the extracted inversion parameter (x) and in turns the relevant cationic distributions, extracted directly from the collected EXAFS signal along with that extracted from the EXAFS fitting, are very close to each other.
3.3 Magnetic behavior of (x)BaTiO3/(1 − x) Li0.5Fe2.5O4 nanocomposite
Figure 9a shows the temperature dependence of the magnetic susceptibility (χM) for the investigated nanocomposites at different magnetic field intensities. It is shown that, a normal ferrimagnetic character is the general trend for all the samples where χM decreases gradually with the temperature until reaching the Curie temperature (TC), after above the sample gives the paramagnetic trend. The χM decreases with increasing the magnetic field intensity as M = χM*H. By increasing the magnetic field intensity, the magnetization increases and χM decreases slightly going hand in hand to cause irreversible domain wall motion. Figure 9b shows the first derivative of the magnetization versus temperature curves for (x)BTO/(1 − x)LFO nanocomposites. Where the minimum point of the first derivative peak represents the Curie temperature (TC) of the samples. It was observed that the Curie temperature was decreased by increasing the BaTiO3 content in the multiferroic except at x = 0.6; the TC was enhanced.
Figure 10a shows the effect of BaTiO3 content (x) on the effective magnetic moment (µeff), while Fig. 10b displays the effect of BaTiO3 content (x) on the TC (K) and χM at room temperature. The variation of the magnetic parameters such as Curie temperature (TC), magnetic susceptibility (χM), and effective magnetic moment (μeff) as a function of BaTiO3 content (x) in (x)BTO/(1 − x)LFO magnetoelectric nanocomposite at room temperature is listed in Table 3. The value of (μeff) increases with the increase in BTO content up to x = 0.6 then decreases. This trend is due to the presence of the non-magnetic perovskite phase and the hetero-interface effects, which influence the magnetic properties of the composite by changing the distribution of the magnetic ions, their spin orientation particularly at interfaces region, and consequently affecting the magnetic interactions [28,29,30].
The room temperature M−H hysteresis loops of (x)BTO/(1 − x)LFO (x = 0.0, 0.2, 0.4, 0.6, 0.8), are shown in Fig. 11. It is found that, the nanocomposites exhibit magnetic hysteresis curves, indicating that the nanocomposites are in ferrimagnetic ordering, arisen from Li-ferrite phase. The origin of the magnetic properties was influenced by the cation distribution (within the magnetic phase), that was calculated and extracted from the EXAFS data analysis and fitting, respectively. It is widely accepted that the inclusion of Li+ and extra Fe3+ in the lattice parameters, the cation arrangement between the tetrahedral and octahedral sites leads to that decreases the Ms to rather low values.
Figure 12a illustrates the dependence of the coercivity (HC) and the saturation magnetization (Ms) on BaTiO3 content (x). It is clearly indicated that, the saturation magnetization of the magnetoelectric nanocomposite decreases linearly with increasing BTO content which may be caused by the existence of diamagnetic (BTO) phase along with the LFO phase. Also, Fig. 12b along with Table 4 show the variation of the remanence ratio (Mr/Ms) with the BTO content, which expresses the squareness of the hysteresis loop. From Table 4, it is obvious also that HC increases with the increase in BTO content (x). Furthermore, the saturation magnetization Ms plays a role in the behavior of HC according to Brown’s relation [31] which is given by; HC ≥ (2K1/μoMs), where K1 is the anisotropy constant, μo is the permeability, Ms is the saturation magnetization. According to Brown’s relation, HC is inversely proportional to Ms which is obeyed the experimental data.
It is reported that the linear decrease in the magnetization of the composite with increasing x is simply due to the NON-MAGNETIC dilution of the magnetic material [32]. In this case, the average magnetization of the composite decreases linearly with increasing x. Similarly, a slight increase in the coercive force can be explained by the decrease in the magnetic interaction between the ferrite particles with an increase in the ratio of the non-magnetic component. Coercivity increased to rather high values; the sample with x = 0.0 has a value of 128.8 Oe while all the samples up to x = 0.8 have a coercivity of ≃ 143 Oe. It is clear that the structure disorder discovered from the EXAFS data analysis affected the observed magnetic behavior of the LFO phase, which in turns controlled the magnetic functionality of the BTO/LFO multiferroic nanocomposite.
4 Conclusions
This research paper displays the results of the study of the two-phasic composite, (x)BaTiO3/(1 − x)Li0.5Fe2.5O4, that was successfully constructed by modified sol–gel auto combustion method at relatively low temperature. Various advanced imaging and characterization techniques were used to study the structural, morphological, and magnetic properties of the synthesized composites: XRD, FTIR, XAFS, HRTEM, AFM and VSM. Interesting results were extracted from the XAFS measurements around Fe K-edge. The reflection of the structure analysis on the magnetic properties was studied and correlated. Modified citrate auto combustion was successful method for obtaining BaTiO3 in nanorods-like shape. Tetra-n-butyl titanate was succeeded in preparing the single phase of BTO nanorods at a relatively low temperature. BaTiO3 improved both TC = 953 K and HC = 134.36 G of the composite particularly at x = 0.6. XAFS results around Fe K-edge confirmed the successful incorporation of the Li atoms into LFO lattice sites. The XANES fingerprint indicated that the Fe ions have a trivalent oxidation character (Fe3+). The structure disorder discovered from the EXAFS data fit explained the observed magnetic behavior of the nanocomposite. The local atomic structure and the cation distribution of the magnetic phase were affecting strongly the observed magnetic performance of the BTO/LFO nanocomposite. Finally, the coupling between the perovskite (BaTiO3) and the spinel (Li0.5Fe2.5O4) phases forming the target multiferroic nanocomposite exerts the shared benefits of the two constituent phases in producing, novel, and more efficient advanced applications particularly in the energy saving field.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. However, the original collected Data are available by contacting the corresponding author. Also, the IFEFFIT code used during the study are available online in accordance with funder data retention policies (the authors provide full citations that include URLs or DOIs.)
References
K. Khan, A.K. Tareen, M. Aslam, R.U.R. Sagar, B. Zhang, W. Huang, A. Mahmood, N. Mahmood, K. Khan, H. Zhang, Z. Guo, Recent progress, challenges, and prospects in two-dimensional photo-catalyst materials and environmental remediation. Nano-Micro Lett. 12, 167 (2020). https://doi.org/10.1007/s40820-020-00504-3
N.G. Imam, M. AbouHasswa, N. Okasha, Synchrotron X-ray absorption fine structure study and dielectric performance of Li0.5Fe2.5O4/BaTiO3 multiferroic. J. Mater. Sci.: Mater. Electron. 32, 21492–21510 (2021). https://doi.org/10.1007/s10854-021-06658-z
N.V. Srihari, K.B. Vinayakumar, K.K. Nagaraja, Magnetoelectric coupling in bismuth ferrite-challenges and perspectives. Coatings 10, 1221 (2020). https://doi.org/10.3390/coatings10121221
M.S. Huzan, M. Fix, M. Aramini, P. Bencok, J.F. Mosselmans, S. Hayama, F.A. Breitner, L.B. Gee, C.J. Titus, M.A. Arrio, A. Jesche, M.L. Baker, Single-ion magnetism in the extended solid-state: insights from X-ray absorption and emission spectroscopy. Chem. Sci. 11, 11801 (2020). https://doi.org/10.1039/d0sc03787g
H. Zeng, T. Tao, Y. Wu, W. Qi, C. Kuang, S. Zhou, Y. Chen, Lithium ferrite (Li0.5Fe2.5O4) nanoparticles as anodes for lithium-ion batteries. RSC Adv. 4, 23145 (2014). https://doi.org/10.1039/c4ra02957g
Y.-P. Fu, Electrical conductivity and magnetic properties of Li0.5Fe2.5−xCrxO4 ferrite. Mater. Chem. Phys. 115, 334–338 (2009). https://doi.org/10.1016/j.matchemphys.2008.12.023
J. Massoudi, D. Bouekkeze, A. Bougoffa, K. Khirouni, E. Dhahri, L. Bessais, Structural, elastic, optical and dielectric properties of Li0.5Fe2.5O4 nanopowders with different particle sizes. Adv. Powder Technol. 31(12), 4714–4730 (2020). https://doi.org/10.1016/j.apt.2020.11.005
X.Y. Zheng, L.J. Riddiford, J.J. Wisser, S. Emori, Y. Suzuki, Ultra-low magnetic damping in epitaxial Li0.5Fe2.5O4 thin films. Appl. Phys. Lett. 117, 092407 (2020). https://doi.org/10.1063/5.0023077
T. Amrillah, A. Hermawan, C.P. Wulandari, A.D. Muthi’Ah, F.M. Simanjuntak, Crafting the multiferroic BiFeO3-CoFe2O4 nanocomposite for next-generation devices: a review. Mater. Manuf. Processes (2021). https://doi.org/10.1080/10426914.2021.1945096
C.M. Costa, K.J. Merazzo, R. Gonçalves, C. Amos, S. Lanceros-Méndez, Magnetically active lithium-ion batteries towards battery performance improvement. iScience 24(6), 102691 (2021). https://doi.org/10.1016/j.isci.2021.102691
A.I. Ali, C.W. Ahn, Y.S. Kim, Enhancement of piezoelectric and ferroelectric properties of BaTiO3 ceramics by aluminium doping. Ceram. Int. 39(6), 6623–6629 (2013). https://doi.org/10.1016/j.ceramint.2013.01.099
A.I. Ali, A. Ullah, K. Park, R. Huh, Y.S. Kim, Ferroelectric enhancement of La-doped BaTiO3 thin films using SrTiO3 buffer layer. Thin Solid Films 551, 127–130 (2014). https://doi.org/10.1016/j.tsf.2013.11.048
N.G. Imam, M. AbouHasswa, G. Aquilanti, S.I. El Dek, N. Okasha, A.A.G. Al Shahawy, Influence of polyethylene glycol on the physical properties of Co0.2Fe2.8O4 nanoparticles used as MRI contrast agent; synchrotron radiation Fe K-edge XAFS. J. Mater. Res. Technol. (2021). https://doi.org/10.1016/j.jmrt.2021.09.143
E.G. Zaki, N.G. Imam, Synchrotron Radiation in Petroleum Applications (Lambert Academic Publishing, Saarbrücken, 2017)
N.G. Imam, G. Aquilanti, A.A. Azab, S.E. Ali, Correlation between structural asymmetry and magnetization in Bi-doped LaFeO3 perovskite: a combined XRD and synchrotron radiation XAS study. J. Mater. Sci.: Mater. Electron. 32, 3361–3376 (2021). https://doi.org/10.1007/s10854-020-05084
M.M. Arman, N.G. Imam, R.L. Portales, S.I. El-Dek, Synchrotron radiation X-ray absorption fine structure and magnetization improvement of A-site Ce3+ doped LaFeO3. J. Magn. Magn. Mater. 513, 167097 (2020). https://doi.org/10.1016/j.jmmm.2020.167097
N. George, J.K. Thomas, H.P. Kumar, M.K. Suresh, V.R. Kumar, P.R.S. Wariar, R. Josed, J. Koshya, Characterization, sintering and dielectric properties of nanocrystalline barium titanate synthesized through a modified combustion process. Mater. Charact. 60, 322 (2009). https://doi.org/10.1016/j.matchar.2008.09.012
M.P. Pileni, Magnetic fluids: fabrication, magnetic properties, and organization of nanocrystals. Adv. Funct. Mater. 11, 323 (2001). https://doi.org/10.1002/1616-3028(200110)11:5%3c323::AID-ADFM323%3e3.0.CO;2-J
M.A. Ahmed, N. Okasha, N.G. Imam, Advanced imaging techniques for characterization of 0.5BaTiO3/0.5Ni0.5Zn0.5Fe2O4 multiferroic nanocomposite. J. Alloys Compd. 557, 130 (2013). https://doi.org/10.1016/j.jallcom.2012.12.036
M.A. Zayed, M.A. Ahmed, N.G. Imam, D.H. El Sherbiny, Analytical characterization of hematite/magnetite ferrofluid nanocomposites for hyperthermia purposes. J. Supercond. Nov. Magn. 29, 2899–2916 (2016). https://doi.org/10.1007/s10948-016-3587
B.H. Tchuigoua, B.T. Sendja, J.M. Mane, G. Aquilanti, J.R. Plaisier, EXAFS study of the temperature dependence of the Debye-Waller factor in ZnSe zincblende compound. Phys. Scr. 95, 045704 (2020). https://doi.org/10.1088/1402-4896/ab6795
B. Ravel, M. Newville, A. Athena, Hephaestus: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005). https://doi.org/10.1107/S0909049505012719
J.J. Rehr, R.C. Albers, Theoretical approaches to x-ray absorption fine structure. Rev. Mod. Phys. 72, 621 (2000). https://doi.org/10.1103/RevModPhys.72.621
A.A.H. El-Bassuony, A comparative study of physical properties of Er and Yb nanophase ferrite for industrial application. J. Supercond. Nov. Magn. (2018). https://doi.org/10.1007/s10948-017-4543-1
D. Carta, G. Mountjoy, G. Navarra, M.F. Casula, D. Loche, S. Marras, A. Corrias, X-ray absorption investigation of the formation of cobalt ferrite nanoparticles in an aerogel silica matrix. J. Phys. Chem. C 111, 6308–6317 (2007). https://doi.org/10.1021/jp0708805
K.K. Kefeni, B.B. Mamba, T.A.M. Msagati, Application of spinel ferrite nanoparticles in water and wastewater treatment: a review. Sep. Purif. Technol. 188, 399–422 (2017). https://doi.org/10.1016/j.seppur.2017.07.015
R.R. Chilwar, A.R. Chavan, M.K. Babrekar, K.M. Jadhav, Impact of trivalent metal ion substitution on structural, optical, magnetic and dielectric properties of Li0.5Fe2.5O4 thin films. Physica B 566, 43–49 (2019). https://doi.org/10.1016/j.physb.2019.04.031
M. Coduri, P. Masala, L. Del Bianco, F. Spizzo, D. Ceresoli, C. Castellano, S. Cappelli, C. Oliva, S. Checchia, M. Allieta, D.-V. Szabo, S. Schlabach, M. Hagelstein, C. Ferrero, M. Scavini, Local structure and magnetism of Fe2O3 maghemite nanocrystals: the role of crystal mansion. Nanomaterials 10, 867 (2020). https://doi.org/10.3390/nano10050867
M.A. Ahmed, N.G. Imam, M.K. Abdelmaksoud, Y.A. Saeid, Magnetic transitions and butterfly-shaped hysteresis of Sm-Fe-Al-based perovskite-type orthoferrite. J. Rare Earth 33, 965 (2015). https://doi.org/10.1016/S1002-0721(14)60513-5
M. Joung, J. Kim, M. Song, J. Choi, S. Nahm, C. Choi, T. Sung, Synthesis of highly tetragonal BaTiO3 nanopowders by a two-step alkoxide–hydroxide route. J. Alloys Compd. 509, 9089–9092 (2011). https://doi.org/10.1016/j.jallcom.2011.06.052
A.M. Abo El Ataa, S.M. Attia, D. El Konya, A.H. Al-Hammadi, Spectral, initial magnetic permeability and transport studies of Li0.5−0.5xCoxFe2.5−0.5xO4 spinel ferrite. J. Magn. Magn. Mater. 295(1), 28–36 (2005). https://doi.org/10.1016/j.jmmm.2004.12.035
S.F. Mansour, N.G. Imam, S. Goda, M.A. Abdo, Constructive coupling between BiFeO3 and CoFe2O4; promising magnetic and dielectric properties. Mater. Res. Technol. 9(2), 1434–1446 (2019). https://doi.org/10.1016/j.jmrt.2019.11.069
Acknowledgements
Access to Synchrotron radiation facilities of Elettra (beamline ‘XAFS’) is appreciated. The Synchrotron XAFS experiment has been supported by 11.1 XAFS Beamline @ Elettra Synchrotron at Trieste (Italy) through SESAME- IAEA-ICTP-Elettra program. The authors appreciate ICTP, IAEA, and EAEA. Neama Imam acknowledges deeply Giuliana Aquilanti, the head of XAFS and XRF beamlines at Elettra and her great group; Luca Olivi, Simone Pollastri, Danilo Oliveiradesouza, and Ilaria Carlomagno. Finally, we all pray for the memory and soul of the late professor M. A. Ahmed.
Funding
Open access funding provided by The Science, Technology &. The author, N.G. Imam acknowledges the support provided from SESAME- IAEA-ICTP-Elettra program for XAFS measurements.
Author information
Authors and Affiliations
Contributions
All authors have contributed, discussed the results and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imam, N.G., AbouHasswa, M., Ali, A.I. et al. Optimization of magnetic properties of BaTiO3/Li0.5Fe2.5O4 multiferroics prepared via modified low-temperature combustion. J Mater Sci: Mater Electron 33, 7945–7959 (2022). https://doi.org/10.1007/s10854-022-07943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-07943-1