Abstract
Glasses from NaCa fluorophosphate doped with varying MnO2 dopant (0.25, 0.50, 1.0%) and melted under different conditions were prepared by normal melting annealing technique. Collective optical, FTIR, photoluminescence, and E.S.R spectral measurements for the various MnO2-doped glasses were done. Optical spectrum of the undoped host fluorophosphate glass reveals only distinct UV absorption which is correlated to contaminated trace iron (Fe3+) ions present as impurities. The MnO2-doped glasses show the same UV absorption beside two additional bands, a small one at 410 nm which is assumed to originate from (Mn2+) ions of spin-forbidden nature and a broad visible and distinct band centered at 500 nm representing the absorption of trivalent manganese (Mn3+) ions of spin-allowed nature. The effects of varying melting conditions reveal obvious changes in the intensities of the two visible bands. FTIR spectra reveal extended vibrational peaks due to phosphate (PO4) groups with sharing of some fluorophosphate (PO3F) groups and the introduction of increasing percent of the dopant that causes minor variations in the FTIR spectra. Photoluminescence spectra reveal a broad emission band centered at about 635 nm and the intensity of the emission band increases with the increase of MnO2 content and is more distinct in glass melted under reducing conditions than that melted at normal condition. E.S.R spectral measurements were carried out to confirm the optical results indicating the existence of measurable divalent Mn2+ ions which increases in reducing conditions. The effects of gamma irradiation (6Mrad dose) on the studied spectral properties were evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphate glasses are considered to be a special system from the three widely studied vitreous glasses beside silicates and borates. They exhibit wide limiting compositions based on the tetrahedral PO4 units within all glasses holding a double bond P = O to compensate for the penta-valency of the phosphorus [1,2,3,4]. Alkali phosphates are highly soluble in water but lead iron phosphate glasses are chemically superior than borosilicate glasses and therefore recommended for burial of some radioactive wastes [4]. The interesting physical properties of some phosphate glasses reveal anomalies especially in the (MgO-P2O5) or (ZnO-P2O5) systems which are related to changes in the coordination number of the respective cation (Mg2+, Zn2+) [3, 5, 6]. The introduction of any of the transition metal or rare-earth ions, fluorides, or heavy metal oxides widens the suitability of the modified phosphate glasses to extend to metal sealing, special optical components, agriglass fertilizer, or as bioactive candidates [5, 7,8,9,10].
Manganese as one of the 3d transition metals is recognized to be able to occur in glasses as both divalent (Mn2+) and trivalent (Mn3+) ions [11, 12]. The ratio of each valence depends on the glass type and its chemical composition and condition of melting [11, 12]. The trivalent manganese ions impart pinkish purple color to glass and exhibit a broad visible band at 450–540 nm which is assumed to be correlated with a spin-allowed 5Eg → 5T2g transition of octahedral symmetry of trivalent manganese [13,14,15]. On the other hand, the divalent manganese (Mn2+) ions exhibit four spin-forbidden weak peaks which can be quietly identified upon increasing the concentration of manganese ion to (8–10%). It is recognized that optical spectral measurements cannot easily distinguished the difference between the absorption peaks due to both the two valences of manganese ions because the spectrum of Mn3+ ions easily obscures or overlaps the absorption due to the weak transitions of spin-forbidden peaks of Mn2+ ions [16, 17].
Therefore, photoluminescence spectroscopy is assumed to be an efficient analytical tool to allow the study of a wide concentration range without interference between Mn3+ and Mn2+ ions due to selectivity towards the divalent Mn2+ ions [15,16,17].
Numerous glass researchers [16,17,18,19,20] have studied the optical and PL spectra of manganese ions in different glass systems and have distinguished the tetrahedrally coordinated and octahedrally coordinated Mn2+ ions by their specific and characteristic positions of the PL emission maximum in the fluorescence spectra.
The present study aims to investigate collective optical, photoluminescence, and FTIR spectral analysis of some prepared host NaCa fluorophosphate glass containing increasing dopant of manganese ions melted under normal, oxidizing, or reducing conditions. The combined spectral analysis is expected to throw more insight about the appearance or abundance of the two valence states of manganese in the studied host glass and the effects of the concentration of Mn ions and condition of melting on the derived spectral results, together with the effects of gamma irradiation (6 Mrad) on the studied spectral properties. A further confirmative study through E.S.R measurements from some selected glasses was followed out and compared with optical results.
2 Experimental details
2.1 Preparation of the glasses
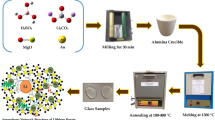
The studied glasses were prepared from laboratory chemicals including sodium dihydrogen orthophosphate (NaH2PO4), CaF2, and MnO2. The batches were melted in covered porcelain crucibles at 900 °C for 60 min including rotating the melts at intervals. The melting was made in a SiC heated furnace (Vecstar, UK). Different additional reagents were added to the batch to induce different conditions of melting including sodium nitrate, sugar, starch, or fine charcoal). The prepared glasses were poured into slightly warmed stainless-steel molds and the samples were immediately transferred to an annealing muffle regulated at 280 °C. The muffle was switched off after 1 h with the samples inside and left to cool to room temperature at a rate of 30 °C/hour.
2.2 Measurements techniques
Polished glass samples of equal thickness (2 mm ± 0.1 were measured for their UV–visible absorption spectra using a recording spectrophotometer (Type JASCO-V-630, Japan) covering the range from 200–1100 nm.
Photoluminescence measurements were carried out at room temperature under the excitation wavelength of 410 nm in spectrophotometer (type JASCO-FP 6500, Japan) equipped with a Xenon arc lamp 150 W as the light source. The scan speed is 500 nm/min with a step interval of 0.5 nm and slit band width 5 nm for both excitation and emission monochromators. Long pass cut on filter was placed on the emission side to avoid second-order light from the monochromator.
FTIR spectral measurements were recorded at room temperature in the wavenumber range 400–4000 cm−1 by a spectrometer (FTIR-4600 Jasco corp., Japan) using the KBr disc technique. Mixtures of 1:100 mg glass powder to KBr were mixed and then subjected to a pressure of 5 tons/cm2 to produce clear discs. At least two measurements were made for each sample. Infrared spectra were corrected for the dark current noises and back ground using the two-point line correction.
Electron spin resonance measurements were carried out at room temperature using a Bruker EMX spectrometer operating in the X-band frequency (9.677 GHz) with field modulation 100 kHz. The microwave power used was 3.196 mW. A powdered glass specimen of 0.3 g was taken, for each sample, in a quartz tube.
An Indian 60Co gamma cell was used for irradiation facility with a dose of 6 Mrad for the studied glasses.
3 Results
3.1 Optical absorption spectra of undoped host glass before and after gamma irradiation
Figures 1 and 2 illustrate the optical spectra of the host NaCa fluorophosphate glass before and after gamma irradiation. The optical spectrum before irradiation reveals distinct UV absorption from 200 to 400 nm and showing three peaks at 215, 240, and 303 nm without any further absorption bands to the rest of measurements at 1100 nm. The optical spectrum of this host glass after irradiation shows extended UV–visible absorption from 200 to about 640 nm and revealing four peaks at 239, 301, 480, and 532 nm.
3.2 Optical absorption spectra of the studied (0.25, 0.5, 1.0% MnO2)-doped glasses melted under different conditions
Figure 3 illustrates the UV–visible absorption spectra of four glass samples containing 0.25% MnO2 after melting at normal atmospheric condition or with added starch, sugar, or charcoal. The detailed spectral absorption spectra are summarized as follows:
(a) The 0.25 MnO2-doped glass melted under normal condition shows a spectrum consisting of distinct and broad UV absorption extending from 200 to about 350 nm with two distinct peaks at 240 and 300 nm with the first peak higher in intensity than the other peak. The spectrum reveals also a broad visible band extending from 400 to 600 nm centered at about 492 nm.
(b) The two glasses melted with starch or sugar show the same strong UV absorption with lower intensities relative to that under normal condition. The rest of the spectral curves show similar data with starch but with sugar, the intensity of the broad band decreases. The glass melted with charcoal shows much lower intensity for UV absorption and the peaks are not clearly identified and the remaining spectrum is similar to that under normal melting but with nearer intensity.
Figure 4 illustrates the optical spectra of the 0.5% MnO2-doped glasses melted at the same four melting conditions. The optical spectra are identified to consist of the distinct strong UV absorption with three peaks (240, 250, 304 nm) and the broad visible band centered at 502 nm.
Figure 5 shows the optical spectra of the glasses containing dopant 1.0% MnO2. The spectral curves are nearer to each other revealing strong UV absorption with 3 distinct peaks at 242, 300, and 322 nm (a small band cited before the cut off) and followed by a broad visible band centered at 504 nm.
Figure 6 shows the optical spectra of the same studied glasses containing 0.25 MnO2 after gamma irradiation (6 Mrad). The optical spectra reveal repetitive curves showing extended UV absorption from 200 to about 350 nm with distinct peaks at 242, 300, and 346 nm and followed by a very broad band extending from about 420 to 650 nm with a peak at 532 nm.
After subjecting to irradiation by gamma rays, the optical spectral curves at Fig. 7 show the extension of the strong UV absorption to about 350 nm with three distinct peaks at 242, 300, and 346 nm and followed by the broad visible band centered at 526 nm.
Figure 8 illustrates the optical spectra of the doped glasses 1% MnO2 after gamma irradiation. The same strong UV absorption with three peaks at 240, 304, and 342 nm and the visible broad band centered at 516 nm.
3.3 FTIR spectra of the studied glasses
Figures 9 and 10 illustrate the FTIR spectra of the prepared undoped and MnO2-doped glasses before and after gamma irradiation.
The IR spectral details appear repetitive in which the derived IR curves seem parallel and keeping all the detailed vibrational peaks within their positions either for the undoped or MnO2-doped samples.
The detailed IR spectrum of the undoped NaCa fluorophosphate is summarized as follows:
-
(a)
A sharp far IR peak is identified at about 540 cm−1.
-
(b)
A medium peak is observed at about 700 cm−1.
-
(c)
A double-split band with two peaks at 920 and 995 cm−1 is identified
-
(d)
A very broad band is observed extending from 1040 to 1750 cm−1 with four distinct peaks at 1150, 1250, 1300, and 1640 cm−1.
-
(e)
Two small peaks are observed at 2840 and 2950 cm−1.
-
(f)
A very broad and distinct near- IR band extending from about 3000 to 3700 cm−1 is centered at 3440 cm−1.
-
(g)
A small peak is observed at about 3750 cm−1.
It is to be mentioned that the intensities of the IR spectral curves are observed to increase with the increase of MnO2 content and remain parallel.
3.4 Photoluminescence spectra
The emission spectra of Mn-doped glasses prepared in different environments with different MnO2 contents are shown in Fig. 11. The emission intensity scales are normalized to the highest intensity scale of Fig. 11c, to reveal the changes of the emission intensity with the MnO2 concentration. The emission spectra show a broad emission band centered at about 620 nm. It is clear that the emission intensity increases with the increase of the MnO2 concentration from 0.25 to 1.0% with red shift of the emission band from about 615 nm for 0.25% MnO2 content to 618 nm for 0.5% and 623 nm for 1.0% MnO2 content. The emission intensity of the prepared glasses at different melting conditions reveals different results for each MnO2 content. At 0.25% MnO2 concentration, the emission intensities of the samples prepared under reducing conditions are lower than that of the sample prepared at normal atmosphere. At 0.5% and 1.0% MnO2 concentrations, the emission intensities of the samples prepared under reducing conditions (sugar and starch) are higher than that of the other samples.
Figure 12 shows the emission spectra of the MnO2-doped glasses after irradiation with gamma rays (6 Mrad). It is clear that the intensities of the emitted bands are lower than the emission intensities before irradiation.
3.5 Electron spin resonance data
Figures 13 and 14 illustrate the E.S.R resonance doped with 0.25, 0.5, and 1% MnO2 before and after gamma irradiation. The E.S.R spectral features indicate that at lower concentrations of 0.25 or 0.5%, only small signals not completely defined are identified while at the highest concentration of 1.0%, a distinct E.S.R signal could be identified and with stability towards gamma irradiation.
4 Discussion
4.1 Interpretation of the distinct UV absorption in the undoped NaCa fluorophosphate glasses
The experimental optical spectral results revealed strong and wide UV absorption spectra with two peaks in the spectrum of the undoped fluorophosphate glass and extended to appear within the spectra of MnO2-doped glasses with various melting conditions.
Sigel and Ginther [21] previously identified distinct UV absorption in commercial silicate glasses and referred to their relation to trace impurities and specifically to traces of iron present in the raw materials during the preparation of the glasses.
A group of glass scientists from Jena University [22,23,24] have for extended times searching for specific optical phosphate, fluorophosphate, and borosilicate glasses to be suitable for distinct recent optical applications. They have studied various glasses doped with TM ions, rare-earth ions, and have reached the conclusion that traces of iron impurities decrease the transmission properties of the various glasses even if present within the ppm level and have recommended the need for ultrapure chemicals to produce special optical glasses suitable for the preparation of lenses, glass lasers, and other optical candidates. They have summarized their extended studies in successive recent review articles [25,26,27,28] (Table 1).
Duffy [29] classified and specified that the strong UV absorption due to traces of iron impurities is due to their charge transfer electron origin and can be easily identified even when the impurity is within the ppm level. Based on previous extended considerations, we assume that the identified strong UV absorption in the optical spectra of the undoped NaCa fluorophosphate glass and extended to all the MnO2-doped glasses spectra is originating from traces of ferric ions within the chemicals used for the preparations of the studied glasses.
4.2 Interpretation of the optical spectra of the MnO2-doped glasses
All the MnO2-doped glasses with variable melting conditions reveal the same strong UV absorption as that for the undoped glass beside two additional spectral features, a small peak at 410 nm and broad visible band centered at about 500 nm. The different conditions of melting show minor variations and the whole optical results can be understood and explained on the following basis:
-
(i)
The combined appearance of the distinct and broad UV absorption with three peaks in the spectra can be related to the same origin through the presence of unavoidable traces ferric ions present as impurities within the chemicals used for the preparation of the glasses.
-
(ii)
The two additional variations in the optical spectra due to the MnO2-doped glasses are attributed to the absorption of manganese ions as referred by the previous published studies on manganese-doped glasses. It is agreed by numerous authors that Mn3+ ions belong to d4 configuration and exhibit a single broad band centered at 480–540 nm due to octahedral coordinated Mn3+ ions while Mn2+ ions belong to d5 configuration and generally exhibit spin-forbidden weak peaks with molar extinction coefficients of these transitions around 250 times lower than that for the spin-allowed transitions of Mn3+ ion [11, 12, 15]. As a result, even traces of Mn3+ ions can mask the appearance of absorption due to Mn2+ ions except when the concentration of manganese ions reaches (8–10%)[11, 12, 15].
Therefore, we conclude that the identified peak at 410 nm is related to Mn2+ ions identified under reducing conditions and the broad band centered at 500 nm is correlated with Mn3+ ions.
4.3 Interpretation of the FTIR spectra
The realization and interpretation of the resultant FTIR spectra are based on the following points [15, 30,31,32,33,34,35]:
-
(i)
It is accepted that FTIR spectra reflect the vibrational bands which represent the structural building groups or to be finger-prints of them.
-
(ii)
The resultant FTIR spectra are evidently depended to the chemical constituents of the glass and specifically to the glass-forming oxides.
-
(iii)
The studied host glass composition consists of P2O5 with main constituent forming structural building (PO4) groups. The other constituents include Na2O as solely modifier oxide, CaF2 which is assumed to be able to form few CaF2 or be composed with phosphate groups as (PO3F) and Ca2+ ions are considered as charge compensators or residence as modifiers.
The dopant (MnO2) is assumed to be acting as modifier oxide.
-
(iv)
The detailed assignments of the host NaCa fluorophosphate glass are introduced as follows [15, 30,31,32,33,34,35]:
-
(a)
The peaks at 400–500 cm−1 are attributed to be related to vibrations of modifier cations (Na+, Ca2+) in their sites.
-
(b)
The peak around 520–560 cm−1 can be related to harmonics or bending vibrations of O = P-O linkages.
-
(c)
The peaks around 760 cm−1 can be related harmonic of P = O bending and to symmetric stretching vibrations of P-O-P units.
-
(d)
The wide absorption bands (800–1200 cm−1) can be correlated with vibrations of metaphosphate groups and to asymmetric stretching of P-O-P groups.
-
(e)
The absorption band at (1250–1290 cm−1) can be related to PO2 asymmetric stretching of the doubly bonded oxygen (P=O).
-
(f)
The absorption band at (1620–1640 cm−1) can correlated with POH bridges or water molecule vibrations.
-
(g)
The extended near-IR absorption from (2850–3500 cm−1) is related to vibrations of POH, OH, or water.
-
(a)
4.4 Interpretation of the photoluminescence spectra
It is recognized that Mn2+ ions emit one band resulted from the transition from the excited state 4T1 (G) to the 6A1 (S) ground state. The energy of the 4T1 (G) state depends strongly on the Mn2+ ions–ligands interactions, which result in variable emission wavelengths of the emitted band. Octahedrally coordinated Mn2+ ions perform a red emission band at about 615 nm due to strong crystal field and in this case the transition is spin-forbidden transition, while the tetrahedrally coordinated Mn2+ ions exhibit green emission, due to weak crystal field and the transition is spin-allowed transition [18, 20, 36]. These assumptions can also be confirmed from Tanabe–Sugano diagram in which Mn2+ belongs to d5 configuration [37]. So, the red emission of the glass samples at about 615 nm is due to 4T1 (G) → 6A1 (S) transition, which indicates the octahedral coordination of Mn2+ ions for the prepared glasses. The broad emission bands reveal the broad distribution of Mn2+ ions sites. The red shift of the emission bands with the increase in the MnO2 content can be interpreted by exchange interactions between the pairs of Mn2+ ions [38] and could also be due to site distribution of Mn2+ ions in the vicinity of ligand fields [39]. The large decrease in emission intensity after irradiation with gamma rays indicates the conversion of Mn2+ ions into Mn3+ ions which is confirmed also by the increase of the absorption band at 532 nm which is characteristic for Mn3+ ions.
4.5 Interpretation of the E.S.R spectral data
The E.S.R spectral details can be understood and explained as follows:
The successive gradient six small signals or six-line pattern identified at low concentration-doped MnO2 glass can be related to several reasons including the small contents of the divalent manganese ions or any related coordination which are able to identify specific signals. Also, it can be assumed to be related to the dipole interactions between the paramagnetic ions coupled by magnetic dipole and the exchange interactions according to the obvious line extension or broadening as suggested by some authors [40, 41]. A similar result has been identified by Konidakis [16] on E.S.R data of mixed strontium–manganese metaphosphate glasses. These authors have assumed that PL spectra are typical for octahedral coordination of the Mn2+ ions. Our PL results are similar to those findings.
The high concentrated MnO2 content (1.0%) reveals distinct and typical signals for Mn2+ ions and the addition of manganese ions in the form of MnO2 seems to support the PL signals and their stability in the phosphate host glasses.
4.6 Interpretation the action of gamma irradiation on the studied glasses.
Radiation processes that take place in glasses are the same that occurs in crystals. It is accepted that multivalent Mn can easily trap electrons or positive holes when irradiated. From the absorption spectra, induced optical spectra are due to the formation of (Mn2+)+-HC. The very small optical absorbed at 400 nm arises from the consumption of Mn2+ by photooxidation [17].
5 Conclusion
Undoped and MnO2-doped (0.25, 0.5 and 1.0%) NaCa fluorophosphate glasses prepared under varying normal, oxidizing, or reducing melting conditions were characterized by combined optical, FTIR, and photoluminescence spectral analysis. A further supplement of E.S.R studies of MnO2-doped samples were conducted to confirm the previous results.
Optical spectral data indicate the appearance of strong UV absorption in the undoped glass and with two additional bands in the spectra of MnO2-doped samples. The first small peak at 410 nm is assumed to originate from spin-forbidden Mn2+ ions, while the characteristic broad band centered around 500 nm originated from Mn3+ ions in characteristic octahedral coordination. The intensities of the absorption bands are identified to change with the concentration of MnO2 and also with the type of melting condition.
FTIR spectra are identified to extend within the mid-region 400–1500 cm−1 revealing the vibrational peaks due mainly to PO4 groups besides the sharing of (PO3F) groups. The observed limited IR spectral changes with concentration of MnO2 dopant or with condition of MnO2 dopant or with condition of melting have been discussed and evaluated.
Photoluminescence spectral results indicate the appearance of PL spectra with limited variations with the percent of MnO2 or condition of melting.
Gamma irradiation is identified to cause some variations of the measured properties and are evaluated.
Data availability
The data of this work are available for any person after publication.
References
S.W. Martin, Review of the structures of phosphate glasses. Eur. J. Solid State Inorg. Chem. 28(1), 163–205 (1991)
G. Guo, Optical and thermal properties of some chemically durable lead phosphate glasses. Glass Technol. 39(4), 138–141 (1998)
R.K. Brow, the structure of simple phosphate glasses. J. Non-Cryst. Solids 263, 1–28 (2000)
D.E. Day, Z. Wu, C.S. Ray, P. Hrma, Chemically durable iron phosphate glass wasteforms. J. Non-Cryst. Solids 241(1), 1–12 (1998)
J.A. Wilder Jr., Glasses and glass ceramics for sealing to aluminum alloys. J. Non-Cryst. Solids 38, 879–884 (1980)
C.E. Smith, R.K. Brow, The properties and structure of zinc magnesium phosphate glasses. J. Non-Cryst. Solids 390, 51–58 (2014)
M.A. Ouis, M.A. Azooz, H.A. ElBatal, Optical and infrared spectral investigations of cadmium zinc phosphate glasses doped with WO3 or MoO3 before and after subjecting to gamma irradiation. J. Non-Cryst. Solids 494, 31–39 (2018)
M.A. Marzouk, I.M. Elkashef, A.M. Fayad, H.A. Elbatal, Photoluminescence, optical and structural properties of Pr3+-doped fluorophosphate glasses and their induced defects by gamma irradiation. J. Mater. Sci. 29, 10561–10572 (2018)
M.A. Ouis, N.A. Ghoneim, H.A. ElBatal, S.I. Shedeed, Evaluation of the suitability of agriglasses containing ZnO for plant fertilization. SILICON 4(1), 61–71 (2012)
H.A. ElBatal, E.M.A. Khalil, Y.M. Hamdy, In vitro behavior of bioactive phosphate glass–ceramics from the system P2O5–Na2O–CaO containing titania. Ceram. Int. 35(3), 1195–1204 (2009)
T. Bates, Ligand field theory and absorption spectra of transition-metal ions in glasses. Modern aspects of the vitreous state 2, 195–254 (1962)
C.R. Bamford, Colour Generation and Control in Glasses, Part of a Collection of Research on Crystalline Glazes Donated (c. 1990) to CSAD by CI Brown. See Shelf 8, Boxes 5, 6, 7 (Elsevier, Amsterdam, 1977).
N.A. Ghoneim, F.A. Moustaffa, A.H. Zahran, F.M.E. El Din, Gamma-ray interaction with lead borate and lead silicate glasses containing manganese. J. Am. Ceram. Soc. 66(6), 447–451 (1983)
N.K. Mohan, M.R. Reddy, C.K. Jayasankar, N. Veeraiah, Spectroscopic and dielectric studies on MnO doped PbO–Nb2O5–P2O5 glass system. J. Alloy. Compd. 458(1–2), 66–76 (2008)
M.A. Marzouk, Y.M. Hamdy, H.A. Elbatal, Photoluminescence and spectral performance of manganese ions in zinc phosphate and barium phosphate host glasses. J. Non-Cryst. Solids 458, 1–14 (2017)
I. Konidakis, C.-P.E. Varsamis, E.I. Kamitsos, D. Möncke, D. Ehrt, Structure and properties of mixed strontium− manganese metaphosphate glasses. J. Phys. Chem. C 114(19), 9125–9138 (2010)
D. Möncke, D. Ehrt, E.I. Kamitsos, Spectroscopic study of manganese-containing borate and borosilicate glasses: cluster formation and phase separation. Phys. Chem. Glasses 54(1), 42–51 (2013)
M. Kawano, H. Takebe, M. Kuwabara, Compositional dependence of the luminescence properties of Mn2+-doped metaphosphate glasses. Opt. Mater. 32(2), 277–280 (2009)
D. Ehrt, Photoluminescence in the UV–VIS region of polyvalent ions in glasses. J. Non-Cryst. Solids 348, 22–29 (2004)
D. Möncke, E.I. Kamitsos, A. Herrmann, D. Ehrt, M. Friedrich, Bonding and ion–ion interactions of Mn2+ ions in fluoride-phosphate and boro-silicate glasses probed by EPR and fluorescence spectroscopy. J. Non-Cryst. Solids 357(14), 2542–2551 (2011)
G. Sigel, R. Ginther, Effect of iron on ultraviolet absorption of high purity soda-Silica glass. Glass Technol. 9(3), 66–000 (1968)
W. Seeber, D. Ehrt, Estimation of deep-UV and UV absorption coefficients of selected trace impurities in glasses. Glass Sci. Technol.-Frankfurt Am Main- 70, 312–315 (1997)
D. Ehrt, P. Ebeling, U. Natura, UV Transmission and radiation-induced defects in phosphate and fluoride–phosphate glasses. J. Non-Cryst. Solids 263, 240–250 (2000)
D. Möncke, D. Ehrt, Irradiation induced defects in glasses resulting in the photoionization of polyvalent dopants. Opt. Mater. 25(4), 425–437 (2004)
D. Möncke, D. Ehrt, L.L. Velli, C.P.E. Varsamis, E.I. Kamitsos, S. Elbers, H. Eckert, Comparative spectroscopic investigation of different types of fluoride phosphate glasses. Phys. Chem. Glasses 48(6), 399–402 (2007)
D. Möncke, Doris Ehrt-Glass chemical research in the spirit of Otto Schott. Phys. Chem. Glasses 56(6), 235–247 (2015)
D. Möncke, Photo-ionization of 3d-ions in fluoride-phosphate glasses. Int. J. Appl. Glas. Sci. 6(3), 249–267 (2015)
D. Ehrt, Deep-UV materials. Adv. Opt. Technol. 7(4), 225–242 (2018)
J.A. Duffy, Charge transfer spectra of metal ions in glass. Phys. Chem. Glasses 38(6), 289–292 (1997)
J. Wong, C.A. Angell, Glass: Structure by Spectroscopy (MDekker, New York, 1976)
A. Abdel-Kader, A.A. Higazy, M.M. Elkholy, Compositional dependence of infrared absorption spectra studies for TeO2-P2O5 and TeO2-P2O5-Bi2O3 glasses. J. Mater. Sci. 2(3), 157–163 (1991)
A.M. Efimov, IR fundamental spectra and structure of pyrophosphate glasses along the 2ZnO· P2O5–2Me2O· P2O5 join (Me being Na and Li). J. Non-Cryst. Solids 209(3), 209–226 (1997)
Y. Moustafa, K. El-Egili, Infrared spectra of sodium phosphate glasses. J. Non-Cryst. Solids 240(1–3), 144–153 (1998)
E. Metwalli, M. Karabulut, D.L. Sidebottom, M. Morsi, R.K. Brow, Properties and structure of copper ultraphosphate glasses. J. Non-Cryst. Solids 344(3), 128–134 (2004)
F.H. El-Batal, Gamma ray interaction with copper-doped sodium phosphate glasses. J. Mater. Sci. 43(3), 1070–1079 (2008)
Q. Yan, Y. Liu, G. Chen, N. Da, L. Wondraczek, Photoluminescence of Mn2+ centers in chalcohalide glasses. J. Am. Ceram. Soc. 94(3), 660–662 (2011)
Y. Tanabe, S. Sugano, On the absorption spectra of complex ions II. J. Phys. Soc. Jpn. 9(5), 766–779 (1954)
C.R. Ronda, T. Amrein, Evidence for exchange-induced luminescence in Zn2SiO4: Mn. J. Lumin. 69(5–6), 245–248 (1996)
C.P. Reddy, V. Naresh, K.T.R. Reddy, Li2OLiFZnF2B2O3P2O5: MnO glasses–Thermal, structural, optical and luminescence characteristics. Opt. Mater. 51, 154–161 (2016)
O. Cozar, D.A. Magdas, I. Ardelean, EPR study of molybdenum-lead-phosphate glasses. J. Non-Cryst. Solids 354(10–11), 1032–1035 (2008)
H.A. El Batal, E.M. Abou Hussein, N.A. El Alaily, F.M. Ezzeldin, Effect of different 3d transition metal oxides on some physical properties of γ-Irradiated Bi2O3-B2O3 glasses: a comparative study. J. Non-Cryst. Solids 528, 119733 (2020)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). We did not receive any funding at all.
Author information
Authors and Affiliations
Contributions
All authors of this work sharing each other as a team group, where all of them did his best effort to manage this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
ElBatal, F.H., Azooz, M.A., Hamdy, Y.M. et al. Optical, photoluminescence, and E.S.R spectral analysis of manganese ions in phosphate glasses melted under various conditions and impact of gamma irradiation. J Mater Sci: Mater Electron 33, 5477–5488 (2022). https://doi.org/10.1007/s10854-022-07738-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-07738-4