Abstract
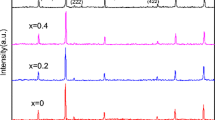
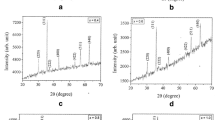
Co1−xCuxFe2O4 (x = 0.0, 0.10, 0.20 and 0.30) nanoparticles with interesting morphologies and magnetic properties were synthesized via thermal decomposition of Cu2+-substituted Co–Fe glycolates. The Cu2+-substituted Co–Fe glycolates were prepared first via a glycolate route which were then calcined at 500 °C to obtain Cu2+-substituted cobalt ferrite nanoparticles. The Cu2+-substituted Co–Fe glycolates and the Cu2+-substituted CoFe2O4 nanoparticles were characterized by various characterization techniques such as XRD, TGA, FT-IR, CHNS, FE-SEM, EDX, TEM, DRS and BET. The morphology of Cu2+-substituted cobalt ferrite nanoparticles could be tailored (hexagonal particles, hexagonal plates and near micro-spherical particles) by varying the concentration of Cu2+ used during the synthesis. Magnetic parameters such as saturation magnetization, coercivity and remanence of the Co1−xCuxFe2O4 nanoparticles were studied at 300 K and 15 K and the observed results have been explained on the basis of concentration of Cu2+, cationic distribution, size and morphology of the nanoparticles.














Similar content being viewed by others
References
S.R. Ahmed, P. Kofinas, Controlled room temperature synthesis of CoFe2O4 nanoparticles through a block copolymer nanoreactor route. Macromolecules 35, 3338–3341 (2002). https://doi.org/10.1021/ma011797x
J.P. Kumar, G.K. Prasad, P.V.R.K. Ramacharyulu, B. Singh, S.A. Roy, Metal ferrite nanoparticles: synthesis, characterization, and studies on decontamination of sulfur mustard. J. Alloy. Compd. 692, 833–840 (2017). https://doi.org/10.1016/j.jallcom.2016.09.083
K.K. Kadyrzhanov, K. Egizbek, A.L. Kozlovskiy, M.V. Zdorovets, Synthesis and properties of ferrite-based nanoparticles. Nanomaterials 9, 1–16 (2019). https://doi.org/10.3390/nano9081079
J. Moyer, C. Vaz, E. Negusse, D. Arena, V. Henrich, Controlling the electronic structure of Co1-xFe2+xO4 thin films through iron doping. Phys. Rev. B: Condens. Matter. Mater. Phys. 83, 035121–035210 (2011). https://doi.org/10.1103/PhysRevB.83.035121
D. Tomar, P. Jeevanandam, Synthesis of cobalt ferrite nanoparticles with different morphologies via thermal decomposition approach and studies on their magnetic properties. J. Alloy. Compd. 843, 155815 (2020). https://doi.org/10.1016/j.jallcom.2020.155815
Z.J. Zhang, Z.L. Wang, B.C. Chakoumakos, J.S. Yin, Temperature dependence of cation distribution and oxidation state in magnetic Mn-Fe ferrite nanocrystals. J. Am. Chem. Soc. 120, 1800–1804 (1998). https://doi.org/10.1021/ja973085l
C. Upadhyay, H.C. Verma, Cation distribution in nanosized Ni–Zn ferrites. J. Appl. Phys. 95, 5746–5751 (2004). https://doi.org/10.1063/1.1699501
M. Houshiar, F. Zebhi, Z. Jafari, A. Alidoust, Z. Askari, Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: a comparison study of size, structural, and magnetic properties. J. Magn. Magn. Mater. 371, 43–48 (2014). https://doi.org/10.1016/j.jmmm.2014.06.059
L.T. Lu, N.T. Dung, L.D. Tung, C.T. Thanh, O.K. Quy, N.V. Chuc, S. Maenosono, N.T.K. Thanh, Synthesis of magnetic cobalt ferrite nanoparticles with controlled morphology, monodispersity and composition: the influence of solvent, surfactant, reductant and synthetic conditions. Nanoscale 8, 3141–3850 (2016). https://doi.org/10.1039/c5nr04266f
M.J. Carey, S. Maat, P. Rice, R.F.C. Farrow, R.F. Marks, A. Kellock, P. Nguyen, B.A. Gurney, Spin valves using insulating cobalt ferrite exchange-spring pinning layers. Appl. Phys. Lett. 81, 1044–1046 (2002). https://doi.org/10.1063/1.1494859
T.E. Torres, A.G. Roca, M.P. Morales, A. Ibarra, C. Marquina, M.R. Ibarra, G.F. Goya, Magnetic properties and energy absorption of CoFe2O4 nanoparticles for magnetic hyperthermia. J. Phys.: Conf. Ser. (2010). https://doi.org/10.1088/1742-6596/200/7/072101
J.H. Lee, Y.M. Huh, Y.W. Jun, J. Seo, J. Jang, H. Song, S. Kim, E. Cho, H. Yoon, J. Suh, J. Cheon, Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 13, 95–99 (2007). https://doi.org/10.1038/nm1467
J. Balavijayalakshmi, N. Suriyanarayanan, R. Jayapraksah, Influence of copper on the magnetic properties of cobalt ferrite nanoparticles. Mater. Lett. 81, 52–54 (2012). https://doi.org/10.1016/j.matlet.2012.04.076
C.C. Naik, S.K. Gaonkar, I. Furtado, A.V. Salker, Effect of Cu2+ substitution on structural, magnetic and dielectric properties of cobalt ferrite with its enhanced antimicrobial property. J. Mater. Sci .Mater. Electron 29, 14746–14761 (2018). https://doi.org/10.1007/s10854-018-9611-1
A. Samavati, M.K. Mustafa, A.F. Ismail, M.H.D. Othman, M.A. Rahman, Copper-substituted cobalt ferrite nanoparticles: structural, optical and antibacterial properties. Mater. Express 6, 473–482 (2016). https://doi.org/10.1166/mex.2016.1338
M.M. Dutta, P. Phukan, Cu-doped CoFe2O4 nanoparticles as magnetically recoverable catalyst for C-N cross-coupling reaction. Catal. Commun. 109, 38–42 (2018). https://doi.org/10.1016/j.catcom.2018.02.014
N. Sanpo, J. Wang, C.C. Berndt, Sol-gel synthesized copper-substituted cobalt ferrite nanoparticles for biomedical applications. J. Nano Res. 25, 110–121 (2013). https://doi.org/10.4028/www.scientific.net/JNanoR.25.110
M. Hadi, K.M. Batoo, A. Chauhan, O.M. Aldossary, R. Verma, Y. Yang, Tuning of structural, dielectric, and electronic properties of Cu doped Co–Zn ferrite nanoparticles for multilayer inductor chip applications. Magnetochemistry 7, 53 (2021). https://doi.org/10.3390/magnetochemistry7040053
M.P. Ghosh, S. Mukherjee, Microstructural, magnetic, and hyperfine characterizations of Cu-doped cobalt ferrite nanoparticles. J. Am. Ceram. Soc. 102, 7509–7520 (2019). https://doi.org/10.1111/jace.16687
A. Anugraha, V.K. Lakshmi, G.S. Kumar, T. Raguram, K.S. Rajni, Synthesis and characterisation of copper substituted cobalt ferrite nanoparticles by sol-gel auto combustion route. IOP Conf. Ser.: Mater. Sci. Eng. (2019). https://doi.org/10.1088/1757-899X/577/1/012059
V.S. Kirankumar, S. Sumathi, Copper and cerium co-doped cobalt ferrite nanoparticles: structural, morphological, optical, magnetic, and photocatalytic properties. Environ. Sci. Pollut. Res. 26, 19189–19206 (2019). https://doi.org/10.1007/s11356-019-05286-9
A. Goyal, S. Kapoor, P. Samuel, V. Kumar, S. Singhal, Facile protocol for reduction of nitroarenes using magnetically recoverable CoM0.2Fe1.8O4 (M = Co, Ni, Cu and Zn) ferrite nanocatalysts. RSC Adv. 5, 51347–51363 (2015). https://doi.org/10.1039/c5ra07190a
H.M.K. Tedjieukeng, P.K. Tsobnang, R.L. Fomekong, P. Etape, P.A. Joy, A. Delcorte, J.N. Lambi, Structural characterization and magnetic properties of undoped and copper-doped cobalt ferrite nanoparticles prepared by the octanoate coprecipitation route at very low dopant concentrations. RSC Adv. 8, 38621–38630 (2018). https://doi.org/10.1039/c8ra08532c
M. Sundararajan, L.J. Kennedy, Photocatalytic removal of rhodamine B under irradiation of visible light using Co1-xCuxFe2O4 (0 ≤ x ≤ 0.5) nanoparticles. J. Environ. Chem. Eng. 5, 4075–4092 (2017). https://doi.org/10.1016/j.jece.2017.07.054
M. Margabandhu, S. Sendhilnathan, S. Senthilkumar, D. Gajalakshmi, Investigation of structural, morphological, magnetic properties and biomedical applications of Cu2+ substituted uncoated cobalt ferrite nanoparticles. Braz. Arch. Biol. Technol. 59, 1–10 (2016). https://doi.org/10.1590/1678-4324-2016161046
M. Hashim, K.S. Alimuddin, S.E. Shirsath, E.M. Mohammed, J. Shah, R.K. Kotnala, H.K. Choi, H. Chung, R. Kumar, Structural, electrical and magnetic properties of Co–Cu ferrite nanoparticles. J. Alloy. Compd. 518, 11–18 (2012). https://doi.org/10.1016/j.jallcom.2011.12.017
M.I.A.A. Maksoud, A. El-ghandour, G.S. El-Sayyad, A.S. Awed, R.A. Fahim, M.M. Atta, A.H. Ashour, A.I. El-Batal, M. Gobara, E.K. Abdel-Khalek, M.M. El-Okr, Tunable structures of copper substituted cobalt nanoferrites with prospective electrical and magnetic applications. J. Mater. Sci. Mater. Electron. 30, 4908–4919 (2019). https://doi.org/10.1007/s10854-019-00785-4
C. Singh, S. Bansal, V. Kumar, K.B. Tikoo, S. Singhal, Encrustation of cobalt doped copper ferrite nanoparticles on solid scaffold CNTs and their comparison with corresponding ferrite nanoparticles: a study of structural, optical, magnetic and photocatalytic properties. RSC Adv. 5, 39052–39061 (2015). https://doi.org/10.1039/c5ra03330f
J. Rodriguez-Carvajal, Recent developments of the program FULLPROF. Commission on powder diffraction (IUCr). Newsletter 26, 12–19 (2001)
A. Abdallah, T. Gaudisson, R. Sibille, S. Nowak, W. Cheikhrouhou-Koubaa, K. Shinoda, M. François, S. Ammar, Structural and magnetic properties of mixed Co-Ln (Ln = Nd, Sm, Eu, Gd and Ho) diethyleneglycolate complexes. Dalton Trans. 44, 16013–16023 (2015). https://doi.org/10.1039/c5dt02346g
U. Sharma, J. Pethaiyan, Sn4+ doping induced novel morphological evolution in zinc titanate heteronanostructures and studies on their optical properties. New J. Chem. 42, 7468–7479 (2018). https://doi.org/10.1039/c7nj04530a
K.M. Choi, H.S. Kil, Y.S. Lee, D. Lim, S. Cho, B. Woo, Preparation and luminescence properties of SrTiO3:Pr3+, Al3+ phosphor from the glycolate method. J. Lumin. 131, 894–899 (2011). https://doi.org/10.1016/j.jlumin.2010.12.020
N. Chakroune, G. Viau, S. Ammar, S.N. Jouini, P. Gredin, M.J. Vaulaya, F. Fievet, Synthesis, characterization and magnetic properties of disk-shaped particles of a cobalt alkoxide: CoII(C2H4O2). New J. Chem. 29, 355–361 (2005). https://doi.org/10.1039/b411117f
G.H. Pan, T. Hayakawa, M. Nogami, Z. Hao, X. Zhang, X. Qu, J. Zhang, Zinc titanium glycolate acetate hydrate and its transformation to zinc titanate microrods: synthesis, characterization and photocatalytic properties. RSC Adv. 5, 88590–88601 (2015). https://doi.org/10.1039/c5ra18292a
X. Jiang, Y. Wang, T. Herricks, Y. Xia, Ethylene glycol-mediated synthesis of metal oxide nanowires. J. Mater. Chem. 14, 695–703 (2004). https://doi.org/10.1039/b313938g
V.G. Pol, Y. Langzam, A. Zaban, Application of microwave superheating for the synthesis of TiO2 rods. Langmuir 23, 11211–11216 (2007). https://doi.org/10.1021/la7020116
K. Krishnan, R.S. Krishnan, Raman and infrared spectra of ethylene glycol. Proc. Indian Acad. Sci.-Sect. A 64, 111–122 (1966). https://doi.org/10.1007/BF03047675
T. Fan, Y. Li, H. Zhang, Surfactant-free solvothermal synthesis of 3D flowerlike iron alkoxide (Fe-EG) micro/nanostructures: structure, formation mechanism, and Fenton oxidation of azo dyes. Ind. Eng. Chem. Res. 56, 11684–11696 (2017). https://doi.org/10.1021/acs.iecr.7b02826
P. Samoila, C. Cojocaru, I. Cretescu, C.D. Stan, V. Nica, L. Sacarescu, V. Harabagiu, Nanosized spinel ferrites synthesized by sol-gel autocombustion for optimized removal of azo dye from aqueous solution. J. Nanomater. 2015, 1–13 (2015). https://doi.org/10.1155/2015/713802
K. Vasundhara, S.N. Achary, S.K. Deshpande, P.D. Babu, S.S. Meena, Size dependent magnetic and dielectric properties of nano CoFe2O4 prepared by a salt assisted gel-combustion method. J. Appl. Phys. 113, 194101 (2013). https://doi.org/10.1063/1.4804946
W.E. Mahmoud, Synthesis and optical properties of Ce-doped ZnO hexagonal nanoplatelets. J. Cryst. Growth 312, 3075–3079 (2010). https://doi.org/10.1016/j.jcrysgro.2010.07.040
F. Tao, Z. Shen, Z. Wang, D. Shu, Q. Liu, Y. Sun, Oxalic acid-assisted hydrothermal synthesis and luminescent of hexagonal NaYF4: Ln3+ (Ln = Sm, Eu, Yb / Er) micro/nanoplates. J. Nanomater. 2017, 1–10 (2017). https://doi.org/10.1155/2017/5320989
J. Lee, E.J. Lee, T.Y. Hwang, J. Kim, Y.H. Choa, Anisotropic characteristics and improved magnetic performance of Ca–La–Co-substituted strontium hexaferrite nanomagnets. Sci. Rep. 10, 1–9 (2020). https://doi.org/10.1038/s41598-020-72608-0
S. Wu, Y. Liu, J. Chang, S. Zhang, Ligand dynamic effect on phase and morphology control of hexagonal NaYF4. CrystEngComm 16, 4472–4477 (2014). https://doi.org/10.1039/c4ce00109e
G. Jia, C. Zhang, S. Ding, L. Wang, L. Lia, H. You, Synthesis and enhanced luminescence of uniform and well-dispersed quasispherical YVO4:Ln3+ (Ln = Eu, Dy) nanoparticles by a solvothermal method. CrystEngComm 14, 573–578 (2012). https://doi.org/10.1039/c1ce05725a
G. Wang, J. Wang, L. Zhao, Q. Zhang, Y. Lu, Facile fabrication of fluorescent inorganic nanoparticles with diverse shapes for cell imaging. Nanomaterials 9, 1–15 (2019). https://doi.org/10.3390/nano9020154
S. Dai, N. Wang, C. Qi, X. Wang, Y. Ma, L. Yang, X. Liu, Q. Huang, C. Nie, B. Hu, X. Wang, Preparation of core-shell structure Fe3O4@C@MnO2 nanoparticles for efficient elimination of U(VI) and Eu(III) ions. Sci. Total Environ. 685, 986–996 (2019). https://doi.org/10.1016/j.scitotenv.2019.06.292
A. Cao, J. Hu, L. Wan, Morphology control and shape evolution in 3D hierarchical superstructures. Sci. China Chem. 55, 2249–2256 (2012). https://doi.org/10.1007/s11426-012-4726-3
L. Poul, N. Jouini, F. Fievet, Layered hydroxide metal acetates (metal = zinc, cobalt, and nickel): elaboration via hydrolysis in polyol medium and comparative study. Chem. Mater. 12, 3123–3132 (2000). https://doi.org/10.1021/cm991179j
S. Anjum, A. Rashid, F. Bashir, M. Pervaiz, R. Zia, Effect of Cu doped nickel ferrites on structural, magnetic and dielectric properties. Mater. Today: Proc. 2, 5559–5567 (2015). https://doi.org/10.1016/j.matpr.2015.11.086
T. Zeeshan, S. Anjum, H. Iqbal, R. Zia, Substitutional effect of copper on the cation distribution in cobalt chromium ferrites and their structural and magnetic properties. Mater. Sci.-Pol. 36, 255–263 (2018). https://doi.org/10.1515/msp-2018-0011
L. Kumar, P. Kumar, A. Narayan, M. Kar, Rietveld analysis of XRD patterns of different sizes of nanocrystalline cobalt ferrite. Int. Nano Lett. 3, 1–12 (2013). https://doi.org/10.1186/2228-5326-3-8
J.A. Gomes, G.M. Azevedo, J. Depeyrot, J. Mestnik-Filho, F.L.O. Paula, F.A. Tourinho, R. Perzynski, Structural, chemical, and magnetic investigations of core-shell zinc ferrite nanoparticles. J. Phys. Chem. C 116, 24281–24291 (2012). https://doi.org/10.1021/jp3055069
S.H. Ng, S.Y. Chew, D.I. Dos Santos, J. Chen, J.Z. Wang, S.X. Dou, H.K. Liu, Hexagonal-shaped tin glycolate particles: a preliminary study of their suitability as Li-ion insertion electrodes. Chem. Asian J. 3, 854–861 (2008). https://doi.org/10.1002/asia.200700321
R. Gaur, P. Jeevanandam, Synthesis of Cd1−xZnxS nanoparticles by a novel thermal decomposition approach and studies on their optical properties. J. Mater. Sci. Mater. Electron. 26, 7223–7231 (2015). https://doi.org/10.1007/s10854-015-3348-x
G. Kiruthigaa, C. Manoharan, C. Raju, S. Dhanapandian, V. Thanikachalam, Synthesis and spectroscopic analysis of undoped and Zn doped SnS2 nanostructure by solid state reaction method. Mater. Sci. Semicond. Process. 26, 533–539 (2014). https://doi.org/10.1016/j.mssp.2014.05.048
D.M. Jnaneshwara, D.N. Avadhani, B. Daruka Prasad, B.M. Nagabhushana, H. Nagabhushana, S. Sharma, S.C. Prashantha, C. Shivakumara, Effect of zinc substitution on the nanocobalt ferrite powders for nanoelectronic devices. J. Alloy. Compd. 587, 50–58 (2014). https://doi.org/10.1016/j.jallcom.2013.10.146
M.A. Almessiere, Y. Slimani, A.D. Korkmaz, N. Taskhandi, M. Sertkol, A. Baykal, S.E. Shirsath, I. Ercan, B. Ozçelik, Sonochemical synthesis of Eu3+ substituted CoFe2O4 nanoparticles and their structural, optical and magnetic properties. Ultrason. Sonochem. 58, 104621 (2019). https://doi.org/10.1016/j.ultsonch.2019.104621
D.M. Jnaneshwara, D.N. Avadhani, B. Daruka Prasad, B.M. Nagabhushana, H. Nagabhushana, S.C. Sharma, C. Shivakumara, J.L. Rao, N.O. Gopal, S.C. Ke, R.P.S. Chakradhar, Electron paramagnetic resonance, magnetic and electrical properties of CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 339, 40–45 (2013). https://doi.org/10.1016/j.jmmm.2013.02.028
M.A. Almessiere, Y. Slimani, A.D. Korkmaz, M. Sertkol, A. Baykal, I. Ercan, B. Özçelik, Sonochemical synthesis of CoFe2-xNdxO4 nanoparticles: structural, optical, and magnetic investigation. J. Supercond. Nov. Magn. 32, 3837–3844 (2019). https://doi.org/10.1007/s10948-019-05147-z
M.A. Almessiere, Y. Slimani, S. Guner, M. Nawaz, A. Baykal, F. Aldakheel, A. Sadaqat, I. Ercan, Effect of Nb substitution on magneto-optical properties of Co0.5Mn0.5Fe2O4 nanoparticles. J. Mol. Struct. 1195, 269–279 (2019). https://doi.org/10.1016/j.molstruc.2019.05.075
D. Sharma, N. Khare, Tuning of optical bandgap and magnetization of CoFe2O4 thin films. App. Phys. Lett. 105, 032404 (2014). https://doi.org/10.1063/1.4890863
M.P. Ghosh, S. Mukherjee, Size variation in nanocrystalline Zn0.2Ni0.8Gd0.05Fe1.95O4 ferrites: exchange bias effect and its correlation with disordered surface spins. Mater. Res. Bull. 125, 110785 (2020). https://doi.org/10.1016/j.materresbull.2020.110785
A. López-Ortega, E. Lottini, C.D.J. Fernández, C. Sangregorio, Exploring the magnetic properties of cobalt-ferrite nanoparticles for the development of a rare-earth-free permanent magnet. Chem. Mater. 27, 4048–4056 (2015). https://doi.org/10.1021/acs.chemmater.5b01034
M.A. Almessiere, Y. Slimani, M. Sertkol, F.A. Khan, M. Nawaz, H. Tombuloglu, E.A. Al-Suhaimi, A. Baykal, Ce–Nd Co-substituted nanospinel cobalt ferrites: an investigation of their structural, magnetic, optical, and apoptotic properties. Ceram. Int. 45, 16147–16156 (2019). https://doi.org/10.1016/j.ceramint.2019.05.133
Acknowledgements
This work was supported by Council of Scientific and Industrial Research (CSIR), Government of India (project number 01(2941)/18/EMR-II). Dimpal Tomar thanks the CSIR for the award of fellowship (JRF/SRF). Thanks are due to Institute Instrumentation Centre, IIT Roorkee for providing different instrumental facilities. The authors are also thankful to the Department of Metallurgical and Materials Engineering, IIT Roorkee for providing the HRTEM facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tomar, D., Jeevanandam, P. Studies on morphological and magnetic properties of Co1−xCuxFe2O4 nanoparticles synthesized via thermal decomposition approach. J Mater Sci: Mater Electron 33, 3514–3534 (2022). https://doi.org/10.1007/s10854-021-07543-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07543-5