Abstract
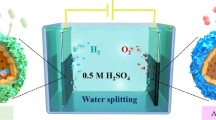
Iron tungstate (FeWO4) nanoparticles were prepared by simple solution combustion technique. The preparation method discloses the first report on the synthesis of iron tungstate nanoparticles. The large-scale synthesis of iron tungstate nanoparticles was achieved and characterized by analytical instruments. The powder XRD patterns authenticated the presence of monoclinic phase of FeWO4 with an average crystalline size of 19nm from Scherrer equation. The optical properties were deliberately assessed with vibrational spectroscopy which predicted the optical band gap of about 2.2 eV. DC and AC conductivity studies suggested that the prepared nanoparticles exhibit excellent conductivity. Furthermore, the semiconducting nature was proved with their temperature-dependent IV curves. The photoconductivity curves serve to be an evident for excellent behavior of light-induced charge carrier’s increment on the prepared iron tungstate nanoparticles. Considerate the interactions between the electrode substrate and nanostructures being an important research in determining the inherent activity of the nanostructures. Photoelectrochemical water splitting towards oxygen evolution was performed on varying the working electrode substrates (Nickel, Platinum, stainless steel, copper) in which higher photon to oxygen conversion rates were observed to be in the increasing order of Pt > SS > Ni > Cu. Herein, the prepared photo anode will revolutionize the design of tandem cells for efficient water splitting process.







Similar content being viewed by others
References
F. Pan, Q. Wang, Molecules 20, 20499 (2015)
N. Kirkaldy, G. Chisholm, J.J. Chen, L. Cronin, Chem. Sci. 9, 1621 (2018)
N.D. Diby, Y. Duan, P.A. Grah, F. Cai, Z. Yuan, J. Mater. Sci. Mater. Electron. 29, 20236 (2018)
S.C. Abbas, J. Wu, Y. Huang, D.D. Babu, G. Anandhababu, M. Arsalan Ghausi, M. Wu, Y. Wang, Int. J. Hydrog. Energy 43, 16–23 (2018)
F.E. Osterloh, Chem. Soc. Rev. 42, 2294 (2013)
R. Ma, S. Wu, H. Yu, S. Chen, A. Sinha, X. Quan, J. Mater. Sci. Mater. Electron. 29, 12700 (2018)
B.J. Rani, G. Ravi, S. Ravichandran, V. Ganesh, F. Ameen, A. Al-Sabri, R. Yuvakkumar, Appl. Nanosci. 8, 1241 (2018)
M.M. Momeni, Y. Ghayeb, J. Mater. Sci. Mater. Electron. 27, 3318 (2016)
L. Liu, J. Hensel, R.C. Fitzmorris, Y. Li, J.Z. Zhang, J. Phys. Chem. Lett. 1, 155 (2010)
J. Huang, S. Liu, L. Kuang, Y. Zhao, T. Jiang, S. Liu, X. Xu, J. Environ. Sci. (China) 25, 2487 (2013)
H. Kim, K. Yong, ACS Appl. Mater. Interfaces 5, 13258 (2013)
V. Nair, C.L. Perkins, Q. Lin, M. Law, Energy Environ. Sci. 9, 1412 (2016)
B.D. Alexander, P.J. Kulesza, I. Rutkowska, R. Solarska, J. Augustynski, J. Mater. Chem. 18, 2298 (2008)
J. Guo, X. Zhou, Y. Lu, X. Zhang, S. Kuang, W. Hou, J. Solid State Chem. 196, 550 (2012)
S. Kang, Y. Li, M. Wu, M. Cai, P.K. Shen, Int. J. Hydrog. Energy 39, 16081 (2014)
N. Goubard-Bretesché, O. Crosnier, G. Buvat, F. Favier, T. Brousse, J. Power Sour. 326, 695 (2016)
H.W. Shim, I.S. Cho, K.S. Hong, W.I. Cho, D.W. Kim, Nanotechnology 21, 465602 (2010)
Y.X. Zhou, H. Bin Yao, Q. Zhang, J.Y. Gong, S.J. Liu, S.H. Yu, Inorg. Chem. 48, 1082 (2009)
Y. Ma, Y. Guo, H. Jiang, D. Qu, J. Liu, W. Kang, Y. Yi, W. Zhang, J. Shi, Z. Han, New J. Chem. 39, 5612 (2015)
C.G. Zoski (ed.), Handbook of Electrochemistry, 1st edn. (Elsevier, Amsterdam, 2007)
J.L. Zhang, M.B. Vukmirovic, Y. Xu, M. Mavrikakis, R.R. Adzic, Angew. Chem.-Int. Ed. 44, 2132–2135 (2005)
A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 1980)
S. Barnartt, J. Electrochem. Soc. 106, 991–994 (1959)
J.D. Benck, B.A. Pinaud, Y. Gorlin, T.F. Jaramillo, PLOS One 9(10), ee107942 (2014)
O. Thoda, G. Xanthopoulou, G. Vekinis, A. Chroneos, Adv. Eng. Mater. 20, 1 (2018)
P. Scherrer, Mathematisch-Physikalische Klasse (Springer, Berlin, 1918), pp. 98–100
J.I. Langford, A.J.C. Wilson, J. Appl. Crystallogr. 11, 102–113 (1978)
V. Uvarov, I. Popov, Mater. Charact. 85, 111 (2013)
K. Sieber, K. Kourtakis, R. Kershaw, K. Dwight, A. Wold, Mater. Res. Bull. 17, 721–725 (1982)
E. Schmidbauer, U. Schanz, F.J. Yu, Phys.: Condens. Matter 3, 5341–5352 (1991)
S. Sagadevan, Appl. Nanosci. 4, 325–329 (2014)
S. Sagadevan, C. Arunseshan, Appl. Nanosci. 4, 179–184 (2014)
S. Sagadevan, J. Podder, I. Das, J. Mater. Sci.: Mater. Electron. 27, 9885–9890 (2016)
J. Lisa Enman, A.E. Vise, M.B. Stevens, S.W. Boettcher, Chem. Phys. Chem. (2019). https://doi.org/10.1002/cphc.201900511
S.M. Abdelbasir, A.M. Elseman, F.A. Harraz, Y.M.Z. Ahmed, S.M. El-Sheikh, M.M. Rashad, New J. Chem. 45, 3150 (2021)
S.M. AlShehri, J. Ahmed, T. Ahamad, P. Arunachalam, T. Ahmad, A. Khan, RSC Adv. 7, 45615 (2017)
M. Athar, M. Fiaz, M. Asim Farid, M. Tahir, M. Adnan Asghar, S. Hassan, M. Hasan, ACS Omega 6(11), 7334–7341 (2021)
F. Shen, Z. Wang, Y. Wang, G. Qian, M. Pan, L. Luo, G. Chen, H. Wei, S. Yin, Nano Res. (2021). https://doi.org/10.1007/s12274-021-3548-z
S.Y. Jeong, J. Song, S. Lee, Appl. Sci. 8, 1388 (2018)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chidambaram, S., Ramachandran, K., Gaidi, M. et al. Solution combustion synthesis of iron tungstate nanoparticles for photoelectrochemical water splitting towards oxygen evolution. J Mater Sci: Mater Electron 33, 9134–9143 (2022). https://doi.org/10.1007/s10854-021-07146-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07146-0