Abstract
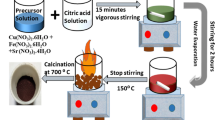
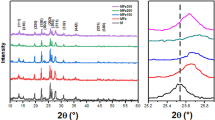
In this study, a ferrate material with a narrow band gap and rapid electron hole separation ability is synthesized for degrading methylene blue from industrial wastewater. Hard ferrite (CoZnFeO4) with octahedral structure is synthesized by solid-phase method from waste ferrous sulfate. The structure of the catalyst was characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTPR) and scanning electron microscopy (SEM). TPR study showed that the oxidation performance of CoZnFeO4 is improved with the addition of cobalt. The total organic carbon removal rate of methylene blue over CoZnFeO4 catalyst is 90% within 60 min. The effects of reaction time, pollutant concentration, catalyst dosage and oxidant concentration on the removal efficiency were optimized. ESI–MS demonstrated the stability of the catalyst for leaching. X-ray diffractometry (XRD) and X-ray energy dispersive spectroscopy (XPS) showed that the catalyst could be reused. These findings provide a low cost and simple strategy for rational design and modulation of catalysts for the industrial degradation of organic pollutants. It not only realizes the use of waste to treat waste, but also accords with the current concept of green chemistry.
























Similar content being viewed by others
References
H. Zollinger, Color chemistry: synthesis, properties and applications of organic dyes and pigments. Leonardo. 22(3/4), 313–314 (1989). https://doi.org/10.2307/1575449
A. Hassani, L. Alidokht, A.R. Khataee, S. Karaca, Optimization of comparative removal of two structurally different basic dyes using coal as a low-cost and available adsorbent. J. Taiwan Inst. Chem. Eng. 45(4), 1597–1607 (2014). https://doi.org/10.1016/j.jtice.2013.10.014
A. Hassani, R.D.C. Soltani, M. Kiransan, S. Kiransan, C. Karaca, A. Khataee, Ultrasound-assisted adsorption of textile dyes using modified nanoclay: central composite design optimization. Korean J. Chem. Eng. 33(1), 178–188 (2016). https://doi.org/10.1007/s11814-015-0106-y
C.H. Chen, Y.H. Liang, W.D. Zhang, ZnFe2O4/MWCNTs composite with enhanced photocatalytic activity under visible-light irradiation. J. Alloys Compd. 501(1), 168–172 (2010). https://doi.org/10.1016/j.jallcom.2010.04.072
W.F. Xui, X.J. Yuan, D.S. Xia, Preparation of nanometer zinc ferrite and its application in water treatment. Technol. Water Treat. (2020). https://doi.org/10.16796/j.cnki.1000-3770.2020.07.002
T. Wu, G. Liu, J. Zhao, Photo-assisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 102(3), 5845–5851 (1998). https://doi.org/10.1021/jp980922c
A.B. Prevot, A. Basso, C. Baiocchi, M. Pazzi, G. Marci, V. Augugliaro, L. Palumisano, E. Pramauro, Analytical control of photocatalytic treatments: degradation of a sulfonated azo dye. Anal. Bioanal. Chem. 378(1), 214–220 (2016). https://doi.org/10.1007/s00216-003-2286-2
D.S. Nair, M. Kurian, Heterogeneous catalytic oxidation of persistent chlorinated organics over cobalt substituted zinc ferrite nanoparticles at mild conditions: reaction kinetics and catalyst reusability studies. J. Environ. Chem. Eng. 5(1), 964–974 (2017). https://doi.org/10.1016/j.jece.2017.01.021
S.B. Somvanshi, P.B. Kharat, T.S. Saraf, Multifunctional nano-magnetic particles assisted viral RNA-extraction protocol for potential detection of COVID-19. Mater. Res. Innov. (2020). https://doi.org/10.1080/14328917.2020.1769350
S.R. Patade, D.D. Andhare, S.B. Somvanshi, Self-heating evaluation of superparamagnetic MnFe2O4 nanoparticles for magnetic fluid hyperthermia application towards cancer treatment. Ceram. Int. (2020). https://doi.org/10.1016/j.ceramint.2020.07.029
P.B. Kharat, S.B. Somvanshi, P.P. Khirade, Induction heating analysis of surface-functionalized nanoscale CoFe2O4 for magnetic fluid hyperthermia toward noninvasive cancer treatment. ACS Omega (2020). https://doi.org/10.1021/acsomega.0c03332
V.L. Ranganatha, S. Pramila, G. Nagaraju, Cost-effective and green approach for the synthesis of zinc ferrite nanoparticles using Aegle Marmelos extract as a fuel: catalytic, electrochemical, and microbial applications. J. Mater. Sci.: Mater. Electron. (2020). https://doi.org/10.1007/s10854-020-04295-6
R.M. Sharma, S. Bansal, S. Singhal, Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M = Cu, Zn, Ni and Co) in the structure. RSC Adv. 5(8), 6006–6018 (2015). https://doi.org/10.1039/C4RA13692F
B. Sheng, Status and development of titanium dioxide industry in 2019. Iron Steel Vanadium Titanium. https://doi.org/10.7513/j.issn.1004-7638.2019.04.001
Y.L. Qiao, L. Ning, Development application, research and production of titanium dioxide in China. Chem. Ind. Eng. Prog. 34(3), 524–535 (1997). https://doi.org/10.1016/S1872-2067(12)60548-8
P. Brax, C. Burrage, Constraining disformally coupled scalar fields. Phys. Rev. D (2014). https://doi.org/10.1103/PhysRevD.90.104009
J.T. Spadaro, L. Isabelle, V. Renganathan, Hydroxyl radical-mediated dradation of azo dyes: evidence for benzene generation. Environ. Sci. Technol. 28(7), 1389–1393 (1994). https://doi.org/10.1021/es00056a031
L. Wolski, M. Ziolek, Insight into pathways of methylene blue degradation with H2O2 over mono and bimetallic Nb, Zn oxides. Appl. Catal. B 224(17), 634–647 (2018). https://doi.org/10.1016/j.apcatb.2017.11.008
D.S. Nair, M. Kurian, Heterogeneous catalytic oxidation of persistent chlorinated organics over cobalt substituted zinc ferrite nanoparticles at mild conditions: reaction kinetics and catalyst reusability studies. J. Environ. Chem. Eng. 5(1), 964–974 (2018). https://doi.org/10.1016/j.jece.2017.01.021
L. Chen, D.H. Ding, C. Liu, H. Cai, Y. Qu, S.J. Yang, Y. Gao, T.M. Cai, Decolorization of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: a comparative study and mechanistic consideration. Chem. Eng. J. 334(15), 273–284 (2018). https://doi.org/10.1016/j.cej.2017.10.040
S.D. Li, H.S. Wang, W.M. Li, X.F. Wu, W.X. Tang, Y.F. Chen, Effect of Cu substitution on promoted benzene oxidation over porous CuCo-based catalysts derived from layered double hydroxide with resistance of water vapor. Appl. Catal. B 166–167, 260–269 (2015). https://doi.org/10.1016/j.apcatb.2014.11.040
S.J. Gregg, K.S.W. Sing, Adsorption, Surface Area and Porosity (Academic Press, London, 1982). https://doi.org/10.1524/zpch.1969.63.14.220
P.D. Yang, D.Y. Zhao, D.I. Margolese, B.F. Chmelka, G.D. Stucky, Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 396(6707), 152–155 (1998). https://doi.org/10.1038/24132
Y.J. Yao, G.D. Wu, F. Lu, S.B. Wang, Y. Hu, J. Zhang, W.Z. Huang, F.Y. Wei, Enhanced photo-Fenton-like process over Z-scheme cofe2o4/g-C3N4 heterostructures under natural indoor light. Environ. Sci. Pollut. Res. 23(21), 21833–21845 (2016). https://doi.org/10.1007/s11356-016-7329-2
M. Sundararajan, V. Sailaja, L.J. Kennedy, J.J. Vijaya, Photocatalytic decolorization of rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: kinetics and mechanism. Ceram. Int. 43(1), 540–548 (2017). https://doi.org/10.1016/j.ceramint.2016.09.191
D.F. Zhang, F.B. Zeng, Visible light-activated cadmium-doped ZnO nanostructured photocatalyst for the treatment of methylene blue dye. J. Mater. Sci. 47(5), 2155–2161 (2012). https://doi.org/10.1007/s10853-011-6016-4
S. Asuha, B. Suyala, S. Zhao, Porous structure and Cr(VI) removal abilities of Fe3O4 prepared from Feurea complex. Mater. Chem. Phys. 129(1–2), 483–487 (2011). https://doi.org/10.1016/j.matchemphys.2011.04.044
Y. Xing, Y.Y. Yin, J.C. Si, M.L. Peng, X.F. Wang, C. Chen, Y.L. Cui, Controllable synthesis and characterization of Fe3O4/Au composite nanoparticles. J. Magn. Magn. Mater. 380, 150–156 (2015). https://doi.org/10.1016/j.jmmm.2014.09.060
A. Zielińska-Jurek, B. Zuzanna, D. Szymon, Design and application of magnetic photocatalysts for water treatment. The effect of particle charge on surface functionality. Catalysts 7(12), 360 (2017). https://doi.org/10.3390/catal7120360
M. Kosmulski, C. Saneluta, Point of zero charge/isoelectric point of exotic oxides: Tl2O3. J. Colloid Interface Sci. 280(2), 544–545 (2004). https://doi.org/10.1016/j.jcis.2004.08.079
M. Chandrika, A.V. Ravindra, C. Rajesh, S.D. Ramarao, S.H. Tu, Studies on structural and optical properties of nano ZnFe2O4 and ZnFe2O4-TiO2 composite synthesized by Co-precipitation route. Mater. Chem. Phys. 230, 107–113 (2019). https://doi.org/10.1016/j.matchemphys.2019.03.059
A. Augustine, A. Pius, Photocatalytic degradation of monocrotophos and chlorpyrifos in aqueous solution using TiO2 under UV radiation. J. Water Process Eng. 7, 94–101 (2015). https://doi.org/10.1016/j.jwpe.2015.06.002
H.H. Yang, B.F. Shi, S.L. Wang, Fe oxides loaded on carbon cloth by hydrothermal process as an effective and reusable heterogenous Fenton catalyst. Catalysts 8(5), 207 (2018). https://doi.org/10.3390/catal8050207
W. Chu, W.K. Choy, The mechanisms of rate enhancing and quenching of trichloroethene photodecay in the presence of sensitizer and hydrogen sources. Water Res. 36(10), 2525–2532 (2002). https://doi.org/10.1016/S0043-1354(01)00471-7
D.F. Ollis, E. Pelizzetti, N. Serpone, Destruction of water contaminants. Environ. Sci. Technol. 9(9), 25 (1991). https://doi.org/10.1021/es00021a001
D.F. Ollis, E. Pelizzetti, N. Serpone, Destruction of water contaminants. Environ. Sci. Technol. (1991). https://doi.org/10.1021/es00021a001
I. Ilisz, K. Foglein, A.J. Dombj, The photochemical behavior of hydrogen peroxide in near UV-irradiated aqueous TiO2 suspensions. J. Mol. Catal. A Chem. 135(1), 55–61 (1998). https://doi.org/10.1016/S1381-1169(97)00296-3
R.M. Gao, J. Stark, D.W. Bahnemann, J. Rabani, Quantum yields of hydroxyl radicals in illuminated TiO2 nanocrystallite layers. J. Photochem. Photobiol. A 148(1–3), 387–391 (2002). https://doi.org/10.1016/s1010-6030(02)00066-7
X.F. Xue, K. Hanna, M. Abdelmoula, N.S. Deng, Adsorption and oxidation of PCP on the surface of magnetite: kinetic experiments and spectroscopic investigations. Appl. Catal. B 89(3–4), 432–440 (2009). https://doi.org/10.1016/j.apcatb.2008.12.024
S.X. Zha, Y. Cheng, Y. Gao, Z.L. Chen, M. Megharej, R. Naidu, Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem. Eng. J. 255, 141–148 (2014). https://doi.org/10.1016/j.cej.2014.06.057
R.A. Doong, W.H. Chang, Photodegradation of parathion in aqueous titanium dioxide and zero valent iron solutions in the presence of hydrogen peroxide. J. Photochem. Photobiol. A 116(3), 221–228 (1998). https://doi.org/10.1016/S1010-6030(98)00292-5
Y.J. Yao, G.D. Wu, F. Lu, S.B. Wang, Y. Hu, J. Zhang, H.Z. Huang, F.Y. Wei, Enhanced photo-Fenton-like process over Z-scheme CoFe2O4/g-C3N4 heterostructures under natural indoor light. Environ. Sci. Pollut. Res. 23(21), 21833–21845 (2016). https://doi.org/10.1007/s11356-016-7329-2
R.M. Sharma, S. Bansal, S. Singhal, Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M=Cu, Zn, Ni and Co) in the structure. RSC Adv. 5(8), 6006–6018 (2015). https://doi.org/10.1039/C4RA13692F
Z.Q. He, C. Gao, M.Q. Qian, Y.Q. Shi, J.M. Chen, S. Song, Electro-Fenton process catalyzed by Fe3O4 magnetic nanoparticles for decolorization of C.I. Reactive Blue 19 in aqueous solution: operating conditions. Influence, and mechanism. Ind. Eng. Chem. Res. 53(9), 3435–3447 (2014). https://doi.org/10.1021/ie403947b
Y.Y. Zhu, Y. Min, V.K. Natarajian, Efficient activation of persulfate by Fe3O4@β-cyclodextrin nanocomposite for removal of bisphenol A. RSC Adv. 8(27), 14879–14887 (2018). https://doi.org/10.1039/C8RA01696H
Z.H. Diao, Q. Wei, P.R. Guo, Photo-assisted degradation of bisphenol A by a novel FeS2@SiO2 microspheres activated persulphate process: synergistic effect, pathway and mechanism. Chem. Eng. J. 349, 683–693 (2018). https://doi.org/10.1016/j.cej.2018.05.132
T.R. Gordon, A.L. Marsh, Temperature dependence of the oxidation of 2-chlorophenol by hydrogen peroxide in the presence of goethite. Catal. Lett. 132(3–4), 349–354 (2009). https://doi.org/10.1007/s10562-009-0125-6
H.Y. Xu, M. Prasad, Y. Liu, Schorl: a novel catalyst in mineral-catalyzed Fenton-like system for dyeing wastewater discoloration. J. Hazard. Mater. 165(1–3), 1186–1192 (2009). https://doi.org/10.1016/j.jhazmat.2008.10.108
Q. Liao, J. Sun, L. Gao, Decolorization of phenol by heterogeneous Fenton reaction using multi-walled carbon nanotube supported Fe2O3 catalysts. Colloid Surf. A 345(1–3), 95–100 (2009). https://doi.org/10.1016/j.colsurfa.2009.04.037
J.H. Sun, S.P. Sun, G.L. Wang, L.P. Qiao, Decolorization of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes Pigm. 74(3), 647–652 (2007). https://doi.org/10.1016/j.dyepig.2006.04.006
F.J. Millero, S. Sotolongo, The oxidation of Fe(II) with H2O2 in seawater. Geochim. Cosmochim. Acta 53(8), 1867–1873 (1989). https://doi.org/10.1016/0016-7037(89)90307-4
S.P. Sun, C.J. Li, J.H. Sun, S.H. Shi, M.H. Fan, Q. Zhan, Decolorization of an azo dye Orange G in aqueous solution by Fenton oxidation process: effect of system parameters and kinetic study. J. Hazard. Mater. 161(2–3), 1052–1057 (2009). https://doi.org/10.1016/j.jhazmat.2008.04.080
S.D. Khairhal, V.S. Shrivastava, Photocatalytic decolorization of chlorpyrifos and methylene blue using α-Bi2O3 nanoparticles fabricated by sol–gel method. SN Appl. Sci. (2019). https://doi.org/10.1007/s42452-019-0761-4
S. Jauhar, S. Singhal, Manganese substituted cobalt ferrites as efficient catalysts for H2O2 assisted decolorization of cationic and anionic dyes: their synthesis and characterization. Appl. Catal. A 486, 210–218 (2014). https://doi.org/10.1016/j.apcata.2014.08.020
S.F. Chen, W. Zhao, W. Liu, H.Y. Zhang, X.L. Yu, Y.H. Chen, Preparation, characterization and activity evaluation of p-n junction photocatalyst-CaFe2O4n-ZnO. Chem. Eng. J. 155, 466–473 (2009). https://doi.org/10.1016/j.cej.2009.07.009
S. Chen, W. Zhao, W. Liu, H. Zhang, X. Yu, Y. Chen, Preparation, characterization and activity evaluation of p-n junction photocatalyst pCaFe2O4/n-Ag3VO4 under visible light irradiation. J. Hazard. Mater. 172(2–3), 1415–1423 (2009). https://doi.org/10.1016/j.jhazmat.2009.08.007
C.H. Chen, Y.H. Lian, W.D. Zhang, ZnFe2O4/MWCNTs composite with enhanced photocatalytic activity under visible-light irradiation. J. Alloys Compd. 501(1), 168–172 (2010). https://doi.org/10.1016/j.jallcom.2010.04.072
R.C.C. Costa, M.D.F.F. Lelis, L.C.A. Oliveira, J.D. Fabvis, J.D. Ardisson, R.R.V.A. Rios, C.N. Silva, R.M. Lago, Remarkable effect of Co and Mn on the activity of FexMxO promoted oxidation of organic contaminants in aqueous medium with HO. Catal. Commun. 4(10), 525–529 (2003). https://doi.org/10.1016/j.catcom.2003.08.002
C.H. Bartholomew, Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 212(1–2), 17–60 (2001). https://doi.org/10.1016/s0926-860x(00)00843-7
P. Forzatti, L. Lietti, Catalyst deactivation. Catal. Today 52(2–3), 165–181 (1999). https://doi.org/10.1016/S0920-5861(99)00074-7
S. Choudhary, A. Bisht, S. Mohapatra, Facile synthesis, morphological, structural, photocatalytic and optical properties of CoFe2O4 nanostructures. SN Appl. Sci. (2019). https://doi.org/10.1007/s42452-019-1665-z
M. Felaez, P. Falaras, V. Likodinos, K. Shea, A. Armah, D.L. Cruz, P.S.M. Dunlop, Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the decolorization of microcystin-LR under visible light irradiation. J. Mol. Catal. A 425, 183–189 (2016). https://doi.org/10.1016/j.molcata.2016.09.035
C.C. Chen, X.Z. Li, W.H. Ma, J.C. Zhao, Effect of transition metal ions on the TiO2-assisted photodecolorization of dyes under visible irradiation: a probe for the interfacial electron transfer process and reaction mechanism. J. Phys. Chem. B 106(2), 318–324 (2002). https://doi.org/10.1021/jp0119025
Q. Liu, Y.R. Guo, Z.H. Chen, Z.G. Zhang, X.M. Fang, Constructing a novel ternary Fe(III)/graphene/g-C3N4, composite photocatalyst with enhanced visible-light-driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B 183, 231–241 (2016). https://doi.org/10.1016/j.apcatb.2015.10.054
N.P. Devi, M. Maisnam, Characterizations of sol-gel synthesized and high energy ball milled spinel nanoferrites: MFe2O4 (M=Li, Ni, Zn, Mn) for nanofluid preparations. Integr. Ferroelectr. 204(1), 133–141 (2020). https://doi.org/10.1080/10584587.2019.1674972
A.K. Srivastava, M. Deepa, N. Bahadur, M.S. Groyat, Influence of Fe doping on nanostructures and photoluminescence of sol-gel derived ZnO. Mater. Chem. Phys. 114(1), 194–198 (2008). https://doi.org/10.1016/j.matchemphys.2008.09.005
A. Manikandan, J.J. Manikandan, M. Sundararajan, M. Bououdina, Optical and magnetic properties of Mg-doped ZnFe2O4 nanoparticles prepared by rapid microwave combustion method. Superlattices Microstruct. 64, 118–131 (2013). https://doi.org/10.1016/j.spmi.2013.09.021
G.J. Srinet, R. Kumar, V. Sajal, Effects of aluminum doping on structural and photoluminescence properties of ZnO nanoparticles. Ceram. Int. 40(3), 4025–4031 (2014). https://doi.org/10.1016/j.ceramint.2013.08.055
B.J. Rani, M. Ravina, B. Saravanakumar, G. Rari, V. Ganesh, S. Ravichandran, R. Yurakkumar, Ferrimagnetism in cobalt ferrite (CoFe2O4) nanoparticles. Nano-Struct. Nano-Objects 14, 84–91 (2018). https://doi.org/10.1016/j.nanoso.2018.01.012
D.D. Dionysiou, M.T. Suidan, I. Baudim, J.M. Laine, Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl. Catal. B: Environ. 50(4), 259–269 (2004). https://doi.org/10.1016/S0926-3373(04)00087-6
A. Khataee, M. Taseidifar, S. Khorram, M. Sheydaei, Preparation of nanostructured magnetite with plasma for decolorization of a cationic textile dye by the heterogeneous Fenton process. J. Taiwan Inst. Chem. Eng. 53, 132–139 (2015). https://doi.org/10.1016/j.jtice.2015.02.023
Y. Lei, C.S. Chen, Y.J. Tu, Y.H. Zhang, Heterogeneous decolorization of organic pollutants by persulfate activated by CuO-Fe3O4: mechanism, stability, and effects of pH and bicarbonate ions. Environ. Sci. Technol. 49(1), 6838–6845 (2015). https://doi.org/10.1021/acs.est.5b00623
Y.J. Yao, G.D. Wu, F. Lu, S.B. Wang, Y. Hu, J. Zhang, W.Z. Huang, F. Wei, Enhanced photo-Fenton-like process over Z-scheme CoFe2O4/g-C3N4 heterostructures under natural indoor light. Environ. Sci. Pollut. Res. 23(21), 21833–21845 (2016). https://doi.org/10.1007/s11356-016-7329-2
M. Li, Y.P. Xiong, X.T. Liu, X.J. Zhang, C. Han, L.P. Guo, Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 7(19), 8920–8930 (2015). https://doi.org/10.1039/C4NR07243J
B. Cai, M.G. Zhao, Y. Ma, Z.Z. Ye, J.Y. Huang, Bioinspired formation of 3D hierarchical CoFe2O4 porous microspheres for magnetic-controlled drug release. ACS Appl. Mater. Interfaces 7(2), 1327–1333 (2015). https://doi.org/10.1021/am507689a
L. Lou, A.E. Nelson, G. Heo, P.W. Major, Surface chemical composition of human maxillary first premolar as assessed by X-ray photoelectron spectroscopy (XPS). Appl. Surf. Sci. 254(21), 6706–6709 (2008). https://doi.org/10.1016/j.apsusc.2008.04.085
J.D. Li, Y.B. Li, L. Zhang, Y. Zuo, Composition of calcium-deficient Na-containing carbonate hydroxyapatite modified with Cu(II) and Zn(II) ions. Appl. Surf. Sci. 254(9), 2844–2850 (2008). https://doi.org/10.1016/j.apsusc.2007.10.030
G.X. Huang, C.Y. Huang, C.W. Yang, P.C. Guo, H.Q. Yu, Decolorization of bisphenol A by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: synergism between Mn and Fe. Environ. Sci. Technol. 51(21), 12611–12618 (2017). https://doi.org/10.1021/acs.est.7b03007
A.S. Albuquerque, M.V.C. Tolentino, J.D. Ardisson, F.C.C. Mouraet, R.D. Mendenca, W.A.A. Macedo, Nanostructured ferrites: structural analysis and catalytic activity. Ceram. Int. 38(3), 2225–2231 (2012). https://doi.org/10.1016/j.ceramint.2011.10.071
S. Lu, G.L. Wang, S. Chen, H.T. Yu, F. Ye, X. Quan, Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for decolorization of organic pollutants. J. Hazard. Mater. 353, 401–409 (2018). https://doi.org/10.1016/j.jhazmat.2018.04.021
C.X. Yang, W.P. Dong, G.W. Cui, Y.Q. Zhao, X.F. Shi, X.Y. Xia, B. Tang, W.L. Wang, Highly efficient photocatalytic decolorization of methylene blue by P2ABSA-modified TiO2 nanocomposite due to the photosensitization synergetic effect of TiO2 and P2ABSA. RSC Adv. 7(38), 23699–23708 (2017). https://doi.org/10.1039/C7RA02423A
A.L. Linsebigler, G. Lu, J.T.Y. Lu, Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95(3), 735–758 (1995). https://doi.org/10.1021/cr00035a013
J.T. Spadaro, L. Isabelle, V. Renganathan, Hydroxyl radical-mediated degradation of azo dyes: evidence for benzene generation. Environ. Sci. Technol. 28(7), 1389–1393 (1994). https://doi.org/10.1021/es00056a031
L. Wolski, M. Ziolek, Insight into pathways of methylene blue degradation with H2O2 over mono and bimetallic Nb, Zn oxides. Appl. Catal. B 224, 634–647 (2018). https://doi.org/10.1016/j.apcatb.2017.11.008
Acknowledgements
This study is financially supported by Sichuan University-Panzhihua City Science and Technology Corporation Special Fund Project for Titanium White by-product Ferrous Sulfate Preparation 500 tons/year Nanometer iron Red Pigment and Co-production Sulfuric Acid Pilot Study Project (No. 2018CDPZH-5), and Sichuan Science and Technology Planning Project (No. 2019YFH0149).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, P., Shu, Y., Zhong, Y. et al. CoZnFeO4 prepared by waste ferrous sulfate as iron source: synthesis, characterization and photocatalytic degradation of methylene blue. J Mater Sci: Mater Electron 32, 20985–21011 (2021). https://doi.org/10.1007/s10854-021-06562-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06562-6