Abstract
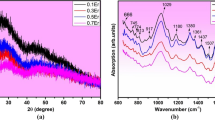
Gd2O3-doped glasses in the B2O3–CaO–Na2O–SrO–P2O5 system were synthesized via melt annealing route and characterized through physical properties. With the replacement of CaO by Gd2O3, the measured values of the density (ds), Gd3+ ions concentration (N), packing density (Pd), oxygen packing density (OPD), Vickers’s hardness (HV), and field strength (F) of the synthesized samples increased, whereas the molar volume (Vm), free volume (Vf), polaron radius (rp), average boron–boron distance (dB–B), and inter-nuclear distance (ri) decreased. The glassy nature of the synthesized samples is confirmed by the X-ray diffraction patterns. The change in the coordination number of boron and the different B–O vibrational bands with the incorporation of gadolinium ions in the investigated glass samples were examined by Raman and FTIR spectroscopy, which supported the presence of BO3, BO4, and GdO4 groups.









Similar content being viewed by others
Code availability
Not applicable.
References
E.I. Kamitsos, G.D. Chryssikos, Borate glass structure by Raman and infrared spectroscopies. J. Mol. Struct. 247, 1–16 (1991)
M. Farouk, A. Samir, F. Metawe, M. Elokr, Optical absorption and structural studies of bismuth borate glasses containing Er3+ ions. J. Non. Cryst. Solids 371, 14–21 (2013)
A.K. Yadav, P. Singh, A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 5(83), 67583–67609 (2015)
V. Venkatramu, D. Navarro-Urrios, P. Babu, C.K. Jayasankar, V. Lavin, Fluorescence line narrowing spectral studies of Eu3+–doped lead borate glass. J. Non. Cryst. Solids 351(10–11), 929–935 (2005)
B. Sumalatha, I. Omkaram, T.R. Rao, C.L. Raju, The structural, optical and magnetic parameter of manganese doped strontium zinc borate glasses. Phys. B Condens. Matter 411, 99–105 (2013)
A.M. Abdelghany, M.A. Ouis, M.A. Azooz, H.A. ElBatal, G.T. El-Bassyouni, Role of SrO on the bioactivity behavior of some ternary borate glasses and their glass ceramic derivatives. Spectrochim. Acta. Part A. 152, 126–133 (2016)
M. Bengisu, Borate glasses for scientific and industrial applications: a review. J. Mater. Sci. 51(5), 2199–2242 (2016)
M. Mariyappan, K. Marimuthu, M.I. Sayyed, M.G. Dong, U. Kara, Effect Bi2O3 on the physical, structural and radiation shielding properties of Er3+ ions doped bismuth sodium fluoroborate glasses. J. Non. Cryst. Solids 499, 75–85 (2018)
S.B. Kolavekar, N.H. Ayachit, G. Jagannath, K. NagaKrishnakanth, S.V. Rao, Optical, structural and Near-IR NLO properties of gold nanoparticles doped sodium zinc borate glasses. Opt. Mater. (Amst) 83, 34–42 (2018)
K.H. Karlsson, K. Fröberg, Structural units in silicate glasses. Chem. Geol. 62(1–2), 1–5 (1987)
P. Naresh et al., Modifier role of ZnO on the structural and transport properties of lithium boro tellurite glasses. J. Non. Cryst. Solids 514, 35–45 (2019)
G. Sangeetha, K.C. Sekhar, A. Hameed, G. Ramadevudu, M.N. Chary, M. Shareefuddin, Influence of CaO on the structure of zinc sodium tetra borate glasses containing Cu2+ ions. J. Non. Cryst. Solids 563, 120784 (2021)
G. El-Damrawi, K. Abd-El-Nour, R.M. Ramadan, Structural and dielectric studies on Na2O–PbO–SiO2 glasses. SILICON 11(1), 495–500 (2019)
Y. Zhou, H. Li, K. Lin, W. Zhai, W. Gu, J. Chang, Effect of heat treatment on the properties of SiO2–CaO–MgO–P2O5 bioactive glasses. J. Mater. Sci. Mater. Med. 23(9), 2101–2108 (2012)
W.C. Lepry, S.N. Nazhat, The anomaly in bioactive sol–gel borate glasses. Mater. Adv. 1(5), 1371–1381 (2020)
M.S. Gaafar et al., Role of neodymium on some acoustic and physical properties of Bi2O3–B2O3–SrO glasses. J. Mater. Res. Technol. 9(4), 7252–7261 (2020)
U. Patel et al., In vitro cellular testing of strontium/calcium substituted phosphate glass discs and microspheres shows potential for bone regeneration. J. Tissue Eng. Regen. Med. 13(3), 396–405 (2019)
R. Divina, K.A. Naseer, K. Marimuthu, Y.S.M. Alajerami, M.S. Al-Buriahi, Effect of different modifier oxides on the synthesis, structural, optical, and gamma/beta shielding properties of bismuth lead borate glasses doped with europium. J. Mater. Sci. Mater. Electron (2020). https://doi.org/10.1007/s10854-020-04662-3
H. Tripathi, C. Rath, A.S. Kumar, P.P. Manna, S.P. Singh, Structural, physicomechanical and in-vitro bioactivity studies on SiO2–CaO–P2O5–SrO–Al2O3 bioactive glasses. Mater. Sci. Eng. 94, 279–290 (2019)
N. Elkhoshkhany, R. Abbas, R. El-Mallawany, A.J. Fraih, Optical properties of quaternary TeO2–ZnO–Nb2O5–Gd2O3 glasses. Ceram. Int. 40(9), 14477–14481 (2014)
H.A. ElBatal et al., In vitro bioactivity behavior of some borophosphate glasses containing dopant of ZnO, CuO or SrO together with their glass-ceramic derivatives and their antimicrobial activity. Silicon 11(1), 197–208 (2019)
B. Samanta, D. Dutta, S. Ghosh, Synthesis and different optical properties of Gd2O3 doped sodium zinc tellurite glasses. Phys. B Condens. Matter 515, 82–88 (2017)
D. Maniu, T. Iliescu, I. Ardelean, I. Bratu, C. Dem, Studies of Borate Vanadate Glasses Using Raman and IR Spectroscopy (Stud. Univ. Babes-Bolyai Phys, Romania, 2001), pp. 366–371
P. Kaur, S. Kaur, G.P. Singh, D.P. Singh, Sm3+ doped lithium aluminoborate glasses for orange-colored visible laser host material. Solid State Commun. 171, 22–25 (2013)
T. Rouxel, Elastic properties of glasses: a multiscale approach. Comptes Rendus Mec. 334(12), 743–753 (2006)
D. Saritha, Y. Markandeya, M. Salagram, M. Vithal, A.K. Singh, G. Bhikshamaiah, Effect of Bi2O3 on physical, optical and structural studies of ZnO–Bi2O3–B2O3 glasses. J. Non. Cryst. Solids 354(52–54), 5573–5579 (2008)
G.P. Singh, S. Kaur, P. Kaur, D.P. Singh, Modification in structural and optical properties of ZnO, CeO2 doped Al2O3–PbO–B2O3 glasses. Phys. B 407(8), 1250–1255 (2012)
M.H.A. Mhareb et al., Impact of Nd3+ ions on physical and optical properties of lithium magnesium borate glass. Opt. Mater. (Amst) 37, 391–397 (2014)
V. Bhatia et al., Mixed transition and rare-earth ion-doped borate glass: structural, optical and thermoluminescence study. J. Mater. Sci. Mater. Electron. 30(1), 677–686 (2019)
S. Karki, C.R. Kesavulu, H.J. Kim, J. Kaewkhao, N. Chanthima, Y. Ruangtaweep, Physical, optical and luminescence properties of B2O3–SiO2–Y2O3–CaO glasses with Sm3+ ions for visible laser applications. J. Lumin. 197, 76–82 (2018)
H. Chandler, Introduction to Hardness Testing (Hardness testing, USA, 1999), pp. 1–13
B. Schrader (ed.), Infrared and Raman Spectroscopy (VCH Publ. Inc., New York, 1995), p. 136
A.M. Abdelghany, Novel method for early investigation of bioactivity in different borate bio-glasses. Spectrochim. Acta. Part A. 100, 120–126 (2013)
S.A. Dalhatu, R. Hussin, K. Deraman, Structural and luminescence properties of Eu3+–doped magnesium sulfide borate glass and crystal. Chinese J. Phys. 54(6), 877–882 (2016)
M. Karabulut, A. Popa, G. Borodi, R. Stefan, An FTIR and ESR study of iron–doped calcium borophosphate glass–ceramics. J. Mol. Struct. 1101, 170–175 (2015)
A.M. Abdelghany, The elusory role of low level doping transition metals in lead silicate glasses. Silicon 2(3), 179–184 (2010)
B. Ashok, K.C. Sekhar, B.S. Chary et al., Physical and structural study of Al2O3–NaBr–B2O3–CuO glasses. Indian J Phys (2021). https://doi.org/10.1007/s12648-021-02048-7
B.N. Meera, J. Ramakrishna, Raman spectral studies of borate glasses. J. Non. Cryst. Solids 159(1–2), 1–21 (1993)
A.A. Osipov, L.M. Osipova, Raman scattering study of barium borate glasses and melts. J. Phys. Chem. Solids 74(7), 971–978 (2013)
A.A. Osipov, L.M. Osipova, B. Hruška, A.A. Osipov, M. Liška, FTIR and Raman spectroscopy studies of ZnO–doped BaO⋅ 2B2O3 glass matrix. Vib. Spectrosc. 103, 102921 (2019)
M.R. Ahmed, B. Ashok, S.K. Ahmmad, A. Hameed, M.N. Chary, M. Shareefuddin, Infrared and Raman spectroscopic studies of Mn2+ ions doped in strontium alumino borate glasses: describes the role of Al2O3. Spectrochim. Acta. Part A. 210, 308–314 (2019)
M.K. Halimah, W.M. Daud, H.A.A. Sidek, A.W. Zaidan, A.S. Zainal, Optical properties of ternary tellurite glasses. Mater. Sci. 28(1), 173–180 (2010)
S. Rada, E. Culea, M. Bosca, M. Culea, P. Pascuta, M. Neumann, Effect of the introduction of gadolinium ions in Boro–tellurite glasses. J. Optoelectron. Adv. Mater. 10(9), 2316–2318 (2008)
Y.B. Saddeek, Elastic properties of Gd3+–doped tellurovanadate glasses using pulse-echo technique. Mater. Chem. Phys. 91(1), 146–153 (2005)
Y. Al-Hadeethi, M.I. Sayyed, Effect of Gd2O3 on the radiation shielding characteristics of Sb2O3–PbO–B2O3–Gd2O3 glass system. Ceram. Int. 46(9), 13768–13773 (2020)
Funding
No funding.
Author information
Authors and Affiliations
Contributions
MAM: conceptualization, methodology, validation, investigation, writing—original draft, writing—review & editing, and visualization. GED: supervision, writing—review & editing. AMA: methodology and formal analysis. MIA: supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Madshal, M.A., El-Damrawi, G., Abdelghany, A.M. et al. Structural studies and physical properties of Gd2O3-doped borate glass. J Mater Sci: Mater Electron 32, 14642–14653 (2021). https://doi.org/10.1007/s10854-021-06022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06022-1