Abstract
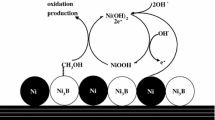
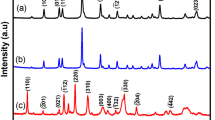
A series of nickel-based materials doping with bismuth element were synthesized by a sol–gel method and investigated as efficient electrocatalysts for methanol electrooxidation in alkaline environment. The physicochemical properties of the materials were well characterized by transmission electron microscope (TEM), scanning electron microscope (SEM), thermogravimetric analysis (TG), X-ray diffraction (XRD), Fourier transform infrared spectra (FT-IR), cyclic voltammetry (CV) and chronoamperometry (CA). The electrochemical measurements illustrated that the introduction of bismuth element can enhance the catalytic activity of NiO catalyst for methanol oxidation reaction. The current density of Ni100Bi1 nano-oxides increased by 30% compared with NiO in 1 M NaOH with 1 M CH3OH solution. The prepared materials exhibited favorable stability for methanol oxidation. Thus, Ni100Bi1 nano-oxides appear to be a promising catalyst for methanol oxidation reaction (MOR).












Similar content being viewed by others
References
X. Peng, D. Chen, X. Yang, D. Wang, M. Li, C.C. Tseng, R. Panneerselvam, X. Wang, W. Hu, J. Tian, Y. Zhao, Microwave-assisted synthesis of highly dispersed PtCu nanoparticles on three-dimensional nitrogen-doped graphene networks with remarkably enhanced methanol electrooxidation. ACS Appl. Mater. Interfaces. 8, 33673–33680 (2016)
C. Li, Z. Yang, X. Liu, Y. Zhang, J. Dong, Q. Zhang, H. Cheng, Enhanced performance of sulfonated poly (ether ether ketone) membranes by blending fully aromatic polyamide for practical application in direct methanol fuel cells (DMFCs). Int. J. Hydrogen Energy. 42, 28567–28577 (2017)
S. Yousefi, D.D. Ganji, Experimental investigation of a passive direct methanol fuel cell with 100 cm2 active areas. Electrochim. Acta 85, 693–699 (2012)
M. Zhang, J. Xie, Q. Sun, Z. Yan, M. Chen, J. Jing, Enhanced electrocatalytic activity of high Pt-loadings on surface functionalized graphene nanosheets for methanol oxidation. Int. J. Hydrogen Energy. 38, 16402–16409 (2013)
K. Mikkelsen, B. Cassidy, N. Hofstetter, L. Bergquist, A. Taylor, D.A. Rider, Block copolymer templated synthesis of core–shell PtAu bimetallic nanocatalysts for the methanol oxidation reaction. Chem. Mater.. 26, 6928–6940 (2014)
A.H.A. Monteverde Videla, D. Sebastián, N.S. Vasile, L. Osmieri, A.S. Aricò, V. Baglio, S. Specchia, Performance analysis of Fe–N–C catalyst for DMFC cathodes: effect of water saturation in the cathodic catalyst layer. Int. J. Hydrogen Energy 41, 22605–22618 (2016)
N. Jung, Y.-H. Cho, M. Ahn, J.W. Lim, Y.S. Kang, D.Y. Chung, J. Kim, Y.-H. Cho, Y.-E. Sung, Methanol-tolerant cathode electrode structure composed of heterogeneous composites to overcome methanol crossover effects for direct methanol fuel cell. Int. J. Hydrogen Energy 36, 15731–15738 (2011)
N. Kakati, J. Maiti, S.H. Lee, S.H. Jee, B. Viswanathan, Y.S. Yoon, Anode catalysts for direct methanol fuel cells in acidic media: do we have any alternative for Pt or Pt-Ru. Chem. Rev. 114, 12397–12429 (2014)
X.-Y. Li, W.-W. Yang, Y.-L. He, T.-S. Zhao, Z.-G. Qu, Effect of anode micro-porous layer on species crossover through the membrane of the liquid-feed direct methanol fuel cells. Appl. Therm. Eng.. 48, 392–401 (2012)
A. Faghri, X. Li, H. Bahrami, Recent advances in passive and semi-passive direct methanol fuel cells. Int. J. Therm. Sci.. 62, 12–18 (2012)
W. Yuan, Z. Zhang, J. Hu, B. Zhou, Y. Tang, Passive vapor-feed direct methanol fuel cell using sintered porous metals to realize high-concentration operation. Appl. Energy 136, 143–149 (2014)
S.D. Sajjad, D. Liu, Z. Wei, S. Sakri, Y. Shen, Y. Hong, F. Liu, Guanidinium based blend anion exchange membranes for direct methanol alkaline fuel cells (DMAFCs). J. Power Sour. 300, 95–103 (2015)
S. Yu, Q. Liu, W. Yang, K. Han, Z. Wang, H. Zhu, Graphene–CeO2 hybrid support for Pt nanoparticles as potential electrocatalyst for direct methanol fuel cells. Electrochim. Acta 94, 245–251 (2013)
L. Song, T. Wang, H. Xue, X. Fan, J. He, In-situ Preparation of pd incorporated ordered mesoporous carbon as efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 191, 355–363 (2016)
Z.L. Zhao, L.Y. Zhang, S.J. Bao, C.M. Li, One-pot synthesis of small and uniform Au@PtCu core–alloy shell nanoparticles as an efficient electrocatalyst for direct methanol fuel cells. Appl. Catal., B. 174–175, 361–366 (2015)
M.M. Mohamed, M. Khairy, S. Eid, Activity and stability studies of titanates and titanate-carbon nanotubes supported Ag anode catalysts for direct methanol fuel cell. J. Power Sour. 304, 255–265 (2016)
R. Ding, L. Qi, M. Jia, H. Wang, Simple hydrothermal synthesis of mesoporous spinel NiCo2O4 nanoparticles and their catalytic behavior in CH3OH electro-oxidation and H2O2 electro-reduction. Catal. Sci. Technol.. 3, 3207–3215 (2013)
L. Qian Jiang, H. Jiang, J. Hou, S. Qi, Wang, G. Sun, Promoting effect of Ni in PtNi Bimetallic electrocatalysts for the methanol oxidation reaction in alkaline media: experimental and density functional theory studies. J. Phys. Chem. C 46, 19714–19722 (2010)
G. Rajeshkhanna, G. Ranga Rao, Micro and nano-architectures of Co3O4 on Ni foam for electro-oxidation of methanol. Int. J. Hydrogen Energy 43, 4706–4715 (2018)
D.E. Pissinis, L.E. Sereno, J.M. Marioli, Characterization of glucose electro-oxidation at Ni and Ni–Cr alloy electrodes. J. Electroanal. Chem. 694, 23–29 (2013)
R.M.A. Hameed, K.M. El-Khatib, Ni–P and Ni–Cu–P modified carbon catalysts for methanol electro-oxidation in KOH solution. Int. J. Hydrogen Energy 35, 2517–2529 (2010)
R.M.A. Hameed, Microwave irradiated Ni–MnOx/C as an electrocatalyst for methanol oxidation in KOH solution for fuel cell application. Appl. Surf. Sci.. 357, 417–428 (2015)
G.-Y. Hou, Y.-Y. Xie, L.-K. Wu, H.-Z. Cao, Y.-P. Tang, G.-Q. Zheng, Electrocatalytic performance of Ni-Ti-O nanotube arrays/NiTi alloy electrode annealed under H2 atmosphere for electro-oxidation of methanol. Int. J. Hydrogen Energy 41, 9295–9302 (2016)
S. Yan, L. Gao, S. Zhang, L. Gao, W. Zhang, Y. Li, Investigation of AuNi/C anode catalyst for direct methanol fuel cells. Int. J. Hydrogen Energy 38, 12838–12846 (2013)
A.S. Bauskar, C.A. Rice, Spontaneously Bi decorated carbon supported Pd nanoparticles for formic acid electro-oxidation. Electrochim. Acta 107, 562–568 (2013)
J. Christoffers, Cerium and bismuth catalysis hand in hand—Synthesis of a eight-membered ring lactam library. Catal. Today 159, 96–99 (2011)
I.G. Casella, M. Contursi, Characterization of bismuth adatom-modified palladium electrodes. Electrochim. Acta 52, 649–657 (2006)
J. Cai, Y. Huang, Y. Guo, Bi-modified Pd/C catalyst via irreversible adsorption and its catalytic activity for ethanol oxidation in alkaline medium. Electrochim. Acta 99, 22–29 (2013)
M.A. Rahman, R. Radhakrishnan, R. Gopalakrishnan, Structural, optical, magnetic and antibacterial properties of Nd doped NiO nanoparticles prepared by co-precipitation method. J. Alloys Compd. 742, 421–429 (2018)
D. Basu, S. Basu, Performance studies of Pd–Pt and Pt–Pd–Au catalyst for electro-oxidation of glucose in direct glucose fuel cell. Int. J. Hydrogen Energy 37, 4678–4684 (2012)
K.H. Ng, S. Lidiyawati, M.R. Somalu, A. Muchtar, H.A. Rahman, Influence of calcination on the properties of nickel oxide-samarium doped ceria carbonate (NiO-SDCC) composite anodes. Procedia Chem. 19, 267–274 (2016)
M.A. Abdel Rahim, R.M. Abdel Hameed, M.W. Khalil, Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sour. 134, 160–169 (2004)
S.N. Azizi, S. Ghasemi, N.S. Gilani, An electrode with Ni(II) loaded analcime zeolite catalyst for the electrooxidation of methanol. Chin. J. Catal. 35, 383–390 (2014)
W. Bing Dong, X. Li, Z. Huang, T. Ali, Z. Zhang, Y. Yang, Hou, Fabrication of hierarchical hollow Mn doped Ni(OH)2 nanostructures with enhanced catalytic activity towards electrochemical oxidation of methanol. Nano Energy 55, 37–41 (2019)
H. Bode, K. Dehmel, J. Witte, Zur kenntnis der nickel-hydroxidelektroded-I.Über das nickel (II)-hydroxidhydrat, Electrochim. Acta 11, 1079–1087 (1966)
D. Singh, Characteristics and effects of g -NiOOH on cell performance and a method to quantify it in nickel electrodes. J. Electrochem. Soc. 145, 116–120 (1998)
B. Norouzi, M. Norouzi, Methanol electrooxidation on novel modified carbon paste electrodes with supported poly(isonicotinic acid) (sodium dodecyl sulfate)/Ni-Co electrocatalysts. J. Solid State Electrochem. 16, 3003–3010 (2012)
H. Cheshideh, F. Nasirpouri, Cyclic voltammetry deposition of nickel nanoparticles on TiO2 nanotubes and their enhanced properties for electro-oxidation of methanol. J. Electroanal. Chem. 797, 121–133 (2017)
N.A.M. Barakat, M.H. El-Newehy, A.S. Yasin, Z.K. Ghouri, S.S. Al-Deyab, Ni&Mn nanoparticles-decorated carbon nanofibers as effective electrocatalyst for urea oxidation. Appl. Catal. A. 510, 180–188 (2016)
X. Song, Q. Sun, L. Gao, W. Chen, Y. Wu, Y. Li, L. Mao, J.-H. Yang, Nickel phosphate as advanced promising electrochemical catalyst for the electro-oxidation of methanol. Int. J. Hydrogen Energy 43, 12091–12102 (2018)
S. Samanta, K. Bhunia, D. Pradhan, B. Satpati, R. Srivastava, Ni and Cu ion-exchanged nanostructured mesoporous zeolite: a noble metal free, efficient, and durable electrocatalyst for alkaline methanol oxidation reaction. Mater. Today Energy 8, 45–56 (2018)
X. Cui, W. Guo, M. Zhou, Y. Yang, Y. Li, P. Xiao, Y. Zhang, X. Zhang, Promoting effect of Co in Ni(m)Co(n) (m + n = 4) bimetallic electrocatalysts for methanol oxidation reaction. ACS Appl. Mater. Interfaces 7, 493–503 (2015)
M. Yu, J. Chen, J. Liu, S. Li, Y. Ma, J. Zhang, J. An, Mesoporous NiCo2O4 nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation. Electrochim. Acta 151, 99–108 (2015)
W. Wang, R. Li, X. Hua, R. Zhang, Methanol electrooxidation on glassy carbon electrode modified with bimetallic Ni(II)Co(II)salen complexes encapsulated in mesoporous zeolite A. Electrochim. Acta 163, 48–56 (2015)
Y.Y. Tong, C.D. Gu, J.L. Zhang, H. Tang, X.L. Wang, J.P. Tu, Thermal growth of NiO on interconnected Ni–P tube network for electrochemical oxidation of methanol in alkaline medium. Int. J. Hydrogen Energy 41, 6342–6352 (2016)
P.R. Jothi, S. Kannan, Enhanced methanol electro-oxidation over in-situ carbon and graphene supported one dimensional NiMoO4 nanorods. J. Power Sour. 277, 350–359 (2015)
Y. Miao, Z. Yang, X. Liu, L. Xu, L. Ouyang, Y. Gu, H. Chang, R. Ouyang, Self-assembly of BiIII ultrathin layer on Pt surface for non-enzymatic glucose sensing. Electrochim. Acta 111, 621–626 (2013)
B. Tang, Y. Lin, Z. Xing, Y. Duan, S. Pan, Y. Dai, J. Yu, J. Zou, Porous coral reefs-like MoS2/nitrogen-doped bio-carbon as an excellent Pt support/co-catalyst with promising catalytic activity and CO-tolerance for methanol oxidation reaction. Electrochim. Acta 246, 517–527 (2017)
F. Wu, Z. Zhang, F. Zhang, D. Duan, Y. Li, G. Wei, S. Liu, Q. Yuan, E. Wang, X. Hao, Exploring the role of cobalt in promoting the electroactivity of amorphous Ni-B nanoparticles toward methanol oxidation. Electrochim. Acta 287, 115–123 (2018)
Y. Huang, J. Cai, Y. Guo, A high-efficiency microwave approach to synthesis of Bi-modified Pt nanoparticle catalysts for ethanol electro-oxidation in alkaline medium. Appl. Catal. B. 129, 549–555 (2013)
Y. Gu, J. Luo, Y. Liu, H. Yang, R. Ouyang, Y. Miao, Synthesis of bimetallic Ni-Cr nano-oxides as catalysts for methanol oxidation in NaOH solution. J. Nanosci. Nanotechnol. 15, 3743–3749 (2015)
Acknowledgements
We acknowledge the National Natural Science Foundation of China (21603143 and 21505092) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, P., Gu, Y., Li, P. et al. Promoting effect of Bi in Ni–Bi oxide electrocatalysts for methanol oxidation reaction. J Mater Sci: Mater Electron 31, 13219–13228 (2020). https://doi.org/10.1007/s10854-020-03873-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03873-y