Abstract
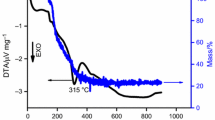
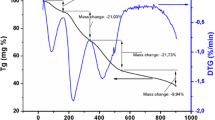
In this work, calcium carbonate (CaCO3), copper oxide (CuO) and titanium oxide (TiO2) were used as precursors to synthesize nano-sized calcium copper titanate CaCu3Ti4O12 (CCTO) powder using environmental friendly and modified sonochemical-assisted process. The precursor mixtures were sonicated at 80 °C for 4 h to get a fully precipitated and homogenous product. A pure phase of CCTO powder was obtained at 900 °C. Various techniques were employed to study the phase formation and structural aspects of the calcined CCTO such as XRD, FTIR, HRTEM, TGA and dielectric spectroscopy. The XRD results confirm the formation single phase with cubic structure of the CCTO phase. The absorption bands in FTIR at 400–700 cm−1, which arise from the mixed vibrations of CuO4 and TiO6 groups, are prevailing in the CCTO structure. Moreover, the HR-TEM micrographs reveal a highly oriented single cubic crystal structure of particle size ~ 4.78 nm. In addition, the dielectric study discloses that the dielectric constant ε′ increased with increasing the calcination temperature up to 900 °C escorted by a decrease of loss factor (tanδ). This can be attributed to the formation of pure CCTO phase and the highly dense microstructure at high temperatures. Giant dielectric constant ε′ up to (106–105) exhibited at low frequency (1–1000 Hz). It is deduced that the optimum calcination temperature of the prepared CCTO must not exceed the temperature range (800–900 °C). Furthermore, the prepared CCTO nanopowder is a promising material for energy storage applications.













Similar content being viewed by others
References
K. Jong-Kuk, K. Nam-Kyoung, P. Byung-Ok, J. Mater. Sci. 35, 4995–4999 (2000)
M. Afqir, M. Elaatmani, A. Zegzouti, A. Oufakir, M. Daoud, J. Mater. Sci. 31, 3048–3056 (2020)
S.J.S. Flora, G. Flora, G. Saxena, Environmental occurrence, health effects and management of lead poisoning, in Lead: Chemistry Analytical Aspects, Environmental Impact and Health Effects, Chap. 4, ed. by S.B. Cascas, J. Sordo (Elsevier Science B.V., Amsterdam, 2006)
U.S. National Library of Medicine, TOXNET—Toxicology Data Network; HSDB—Hazardous Substances Data Bank; Barium Compounds, Accessed Nov. 4, (2015).
M.A. Subramanian, D. Li, N. Duan, B.A. Reisner, A.W. Sleight, J. Solid State Chem. 151, 323–325 (2000)
J. Liu, C.G. Duan, W.N. Mei, R.W. Smith, J.R. Hardy, J. Appl. Phys. 98, 093703-1–093703-5 (2005)
W. Li, R.W. Schwartz, Phys. Rev. B 75, 012104-1–012104-4 (2007)
P. Lunkenheimer, V. Bobnar, A.V. Pronin, A.I. Ritus, A.A. Volkov, A. Loidl, Phys. Rev. B 66, 052105-1–052105-4 (2002)
P. Lunkenheimer, R. Fichtl, S.G. Ebbinghaus, A. Loidl, Phys. Rev. B 70, 172102-1–172102-4 (2004)
H.J. Hwang, K. Niihara, J. Mater. Sci. 33, 549–558 (1998)
T. Ishii, M. Endo, K. Masuda, K. Ishida, Appl. Phys. Lett. 102, 062901-1–062901-4 (2013)
P. Liu, Y. Lai, Y. Zeng, S. Wu, Z. Huang, J. Han, J. Alloys Comp. 650, 59–64 (2015)
J. Liu, R.W. Smith, W.N. Mei, Chem. Mater. 19, 6020–6024 (2007)
Z. Yang, Y. Zhang, R. Xiong, J. Shi, Mater. Res. Bull. 48, 310–314 (2013)
S.M. Moussa, B.J. Kennedy, Mater Res B 36(13-14), 2525–2529 (2001)
N. Wongpisutpaisan, N. Vittayakorn, A. Ruangphanit, Pecharapa W. 149, 56–60 (2013)
Shengtao, L., Hui, W., Chunjiang, L., Yang, Y., Jianying, L.: Dielectric properites of Al-doped CaCu3Ti4O12 ceramics by co-precipitation method. In: Proceed. Int. Conf. Electrical Insulating Materials (ISEIM), IEEE, pp. 23–26 (2011)
M.C. Sonia, P. Kumar, Process Appl. Ceram. 11, 154–159 (2017)
M.M. Ahmad, E. Al-Libidi, A. Al-Jaafari, S. Ghazanfar, K. Yamada, Appl. Phys. A 116, 1299–1306 (2014)
W.X. Yuana, S.K. Harka, W.N. Meib, J. Ceram. Process. Res. 10, 696–699 (2009)
V. Saez, T.J. Mason, Molecules 14, 4284–4299 (2009)
G. Cravotto, P. Cintas, Chem. Soc. Rev. 35, 180–196 (2006)
G. Kianpour, M. Salavati-Niasari, H. Emadi, Ultrason. Sonochem. 20, 418–424 (2013)
A. Aronne, M. Turco, G. Bagnasco, P. Pernice, M. Di Serio, N.J. Clayden, E. Marenna, E. Fanelli, Chem. Mater. 17, 2081–2090 (2005)
S. Guillemet-Fritsch, T. Lebey, M. Boulos, Durand B. J. Eur. Ceram. Soc. 26, 1245–1257 (2006)
S. Jin, H. Xia, Y. Zhang, J. Guo, J. Xu, Mater. Lett. 61, 1404–1407 (2007)
S. Singh, S.B. Krupanidhi, Phys. Letter A 367, 356–359 (2007)
S.A. Gad, G.M. El Komy, A.M. Moustafa, A.A. Ward, Indian J. Phys. 93, 1009–1018 (2019)
P. Thomas, K. Dwarakanath, K. Varma, T. Kutty, J. Therm. Anal. Calorim. 95, 267–272 (2008)
P. Thomas, K. Dwarakanath, K.B.R. Varma, T.R.N. Kutty, J. Phys. Chem. Solids 69, 2594–2604 (2008)
T.B. Adams, D.C. Sinclair, A.R. West, Adv. Mater. 14, 1321–1323 (2002)
H. Doweidar, K. El-Egili, R. Ramadan, M. Al-Zaibani, J. Non-Crystal. Solids 466, 37–44 (2017)
A.F.L. Almeida, P.B.A. Fechine, M.P.F. Graca, M.A. Valente, A.S.B. Sombra, J. Mater. Sci. Mater. Electron. 20, 163–170 (2009)
L. Bayarjargal, C.-J. Fruhner, N. Schrodt, B. Winkler, Phys. Earth Planet. Inter. 281, 31–45 (2018)
M. Todaro, A. Alessi, L. Sciortino, S. Agnello, M. Cannas, F.M. Gelardi, G. Buscarino, J. Spectrosc. (2016). https://doi.org/10.1155/2016/8074297
N. Kolev, R.P. Bontchev, A.J. Jacobson, V.N. Popov, V.G. Hadjiev, A.P. Litvinchuk, M.N. Iliev, Phys. Rev. B 66(13), 132102 (2002)
L. Singh, I.W. Kim, B.C. Sin, K.D. Mandal, U.S. Rai, A. Ullah, H. Chung, Y. Lee, RSC Adv. 4(95), 52770–52784 (2014)
S.A. Gad, A.M. Moustafa, A.A. Ward, J. Inorg. Organomet. Polym. 25, 1077–1087 (2015)
F.D. Morrison, D.C. Sinclair, A.R. West, J. Am. Ceram. Soc. 84, 474–476 (2001)
J. Li, K. Cho, N. Wu, A. Ignatiev, IEEE Trans. 11, 534–541 (2004)
Acknowledgement
This project was supported financially by the Science Technology Development Fund (STDF) Egypt, Grant No. 15184.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramadan, R.M., Labeeb, A.M., Ward, A.A. et al. New approach for synthesis of nano-sized CaCu3Ti4O12 powder by economic and innovative method. J Mater Sci: Mater Electron 31, 9065–9075 (2020). https://doi.org/10.1007/s10854-020-03490-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03490-9