Abstract
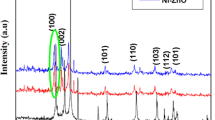
In the present work, we investigate the effect of Ni doping on the crystallite size (Dhkl), optical band gap (\(E_{\text{g}}^{\text{opt}}\)), Photoluminescence emission (PL) behavior, as well as the photocatalytic degradation efficiency of methylene blue (MB) by ZnS nanoparticles (NPs) catalyst. Undoped and Ni-doped ZnS NPs at Ni concentrations of 2, 4, 6, 8, and 10% are successfully synthesized with average Dhkl from 2.75 to 3.76 nm by the sonochemical technique. X-ray diffraction (XRD) patterns and high-resolution transmission electron microscope (HRTEM) images of all samples exhibit pure zinc-blende type of ZnS cubic structure. The increase in Ni content up to 4% results in an increase in the Dhkl and unit cell volume (V) accompanied by a decrease in \(E_{\text{g}}^{\text{opt}}\). Meanwhile, a further increase in Ni content above 4% leads to a decrease in Dhkl and V and an increase in \(E_{\text{g}}^{\text{opt}}\). The deconvoluted PL emission spectrum of the undoped sample at the excitation wavelength (λex) of 325 nm reveals emission bands centered at 3.41, 3.16, 2.89, and 2.26 eV, which are red-shifted with increasing λex to 370 nm. It is observed that the PL emission intensity is quenched with increasing Ni content without any noticeable change in the PL peak position. Ni-doped ZnS catalyst with 2% Ni exhibits maximum photo-degradation efficiency of 52.23% with a rate constant of 0.00396 min−1. The obtained results demonstrate that Ni doping can tune the optical band gap and photocatalytic efficiency of ZnS NPs that make it applicable for many optoelectronic applications.












Similar content being viewed by others
References
M. Afzaal, M.A. Malik, P. O’Brien, Chemical routes to chalcogenide materials as thin films or particles with critical dimensions with the order of nanometres. J. Mater. Chem. 20(20), 4031–4040 (2010)
X. Fang et al., ZnS nanostructures: from synthesis to applications. Prog. Mater Sci. 56(2), 175–287 (2011)
S. Biswas, S. Kar, Fabrication of ZnS nanoparticles and nanorods with cubic and hexagonal crystal structures: a simple solvothermal approach. Nanotechnology 19(4), 45710 (2008)
L. Dong, Y. Chu, Y. Zhang, Microemulsion-mediated solvothermal synthesis of ZnS nanowires. Mater. Lett. 61(23–24), 4651–4654 (2007)
T. Kang et al., Synthesis and magnetic properties of single-crystalline Mn/Fe-doped and Co-doped ZnS nanowires and nanobelts. J. Phys. Chem. C 113(14), 5352–5357 (2009)
X. Duan, Y. Huang, R. Agarwal, C.M. Lieber, Single-nanowire electrically driven lasers. Nature 421(6920), 241 (2003)
M. Bredol, J. Merikhi, ZnS precipitation: morphology control. J. Mater. Sci. 33(2), 471–476 (1998)
V. Stanić, T.H. Etsell, A.C. Pierre, R.J. Mikula, Sol-gel processing of ZnS. Mater. Lett. 31(1–2), 35–38 (1997)
T.T. Hoa, L. Van Vu, T.D. Canh, N.N. Long, Preparation of ZnS nanoparticles by hydrothermal method. J. Phys: Conf. Ser. 187, 012081 (2009)
X. Zhou, Q. Yang, H. Wang, F. Huang, J. Zhang, S. Xu, Effects of Ni2+concentration and vacuum annealing on structure, morphology and optical properties of Ni doped ZnS nanopowders synthesized by hydrothermal method. Adv. Powder Technol. 29(4), 977–984 (2018)
X.H. Liao, J.J. Zhu, H.Y. Chen, Microwave synthesis of nanocrystalline metal sulfides in formaldehyde solution. Mater. Sci. Eng., B 85(1), 85–89 (2001)
Y. Zhao, J.M. Hong, J.J. Zhu, Microwave-assisted self-assembled ZnS nanoballs. J. Cryst. Growth 270(3–4), 438–445 (2004)
H. Yang, C. Huang, X. Su, A. Tang, Microwave-assisted synthesis and luminescent properties of pure and doped ZnS nanoparticles. J. Alloys Compd. 402(1–2), 274–277 (2005)
E.K. Goharshadi, S.H. Sajjadi, R. Mehrkhah, P. Nancarrow, Sonochemical synthesis and measurement of optical properties of zinc sulfide quantum dots. Chem. Eng. J. 209, 113–117 (2012)
N.A. Dhas, A. Zaban, A. Gedanken, Surface synthesis of zinc sulfide nanoparticles on silica microspheres: sonochemical preparation, characterization, and optical properties. Chem. Mater. 11(3), 806–813 (1999)
S. Senthilkumaar, R.T. Selvi, Formation of hexagonal shaped wurtzite zinc sulphide nano rods. Appl. Phys. A 94(1), 123–129 (2009)
W.B. McNamara III, Y.T. Didenko, K.S. Suslick, Sonoluminescence temperatures during multi-bubble cavitation. Nature 401(6755), 772 (1999)
A. Datta, S. Biswas, S. Kar, S. Chaudhuri, Multicolor luminescence from transition metal ion (Mn2+ and Cu2+) doped ZnS nanoparticles. J. Nanosci. Nanotechnol. 7(10), 3670–3676 (2007)
T.T.Q. Hoa, N.D. The, S. McVitie, N.H. Nam, T.D. Canh, N.N. Long, Optical properties of Mn-doped ZnS semiconductor nanoclusters synthesized by a hydrothermal process. Opt. Mater. (Amst) 33(3), 308–314 (2011)
G. Murugadoss, Luminescence properties of co-doped ZnS:Ni, Mn and ZnS:Cu, Cd nanoparticles. J. Lumin. 132(8), 2043–2048 (2012)
L. Liu, L. Yang, Y. Pu, D. Xiao, J. Zhu, Optical properties of water-soluble Co2+: ZnS semiconductor nanocrystals synthesized by a hydrothermal process. Mater. Lett. 66(1), 121–124 (2012)
H.Z. Khafri, M. Ghaedi, A. Asfaram, M. Safarpoor, Synthesis and characterization of ZnS: Ni NPs loaded on AC derived from apple tree wood and their applicability for the ultrasound assisted comparative adsorption of cationic dyes based on the experimental design. Ultrason. Sonochem. 38, 371–380 (2017)
D. Li, J. Qin, Q. Xu, G. Yan, A room-temperature phosphorescence sensor for the detection of alkaline phosphatase activity based on Mn-doped ZnS quantum dots. Sens. Actuators B Chem. 274, 78–84 (2018)
L. Wang et al., Synthesis of Mn-doped ZnS microspheres with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 391, 557–564 (2017)
C.J. Chang, P.Y. Chao, Efficient photocatalytic hydrogen production by doped ZnS grown on Ni foam as porous immobilized photocatalysts. Int. J. Hydrog. Energy 44, 20805–20814 (2018)
M.E. Pacheco, C.B. Castells, L. Bruzzone, Mn-doped ZnS phosphorescent quantum dots: coumarins optical sensors. Sens. Actuators B Chem. 238, 660–666 (2017)
B. Poornaprakash, U. Chalapathi, S.V.P. Vattikuti, Compositional, morphological, structural, microstructural, optical, and magnetic properties of Fe Co, and Ni doped ZnS nanoparticles. Appl. Phys. A 123(4), 275 (2017)
R.S. Kumar, V. Veeravazhuthi, N. Muthukumarasamy, M. Thambidurai, Superlattices and microstructures effect of nickel doping on structural and optical properties of ZnS nanoparticles. Superlattices Microstruct. 86, 552–558 (2015)
D. Jiang et al., Synthesis and luminescence properties of core/shell ZnS:Mn/ZnO nanoparticles. Nanoscale Res. Lett. 4(1), 78 (2009)
X. Li, C. Hu, H. Liu, J. Xu, B. Wan, X. Wang, ZnS nanoparticles self-assembled from ultrafine particles and their highly photocatalytic activity. Phys. E Low-dimens. Syst. Nanostructures 43(5), 1071–1075 (2011)
S. Kohtani, E. Yoshioka, H. Miyabe, Photocatalytic Hydrogenation on Semiconductor Particles, in Hydrogenation (Intech, Rijeka, 2012)
S.H. Mohamed, Photocatalytic, optical and electrical properties of copper-doped zinc sulfide thin films. J. Phys. D Appl. Phys. 43(3), 35406 (2010)
H.R. Rajabi, M. Farsi, Effect of transition metal ion doping on the photocatalytic activity of ZnS quantum dots: synthesis, characterization, and application for dye decolorization. J. Mol. Catal. A: Chem. 399, 53–61 (2015)
J. Kaur, M. Sharma, O.P. Pandey, Photoluminescence and photocatalytic studies of metal ions (Mn and Ni) doped ZnS nanoparticles. Opt. Mater. (Amst) 47, 7–17 (2015)
A.A. Othman, M.A. Ali, E.M.M. Ibrahim, M.A. Osman, Influence of Cu doping on structural, morphological, photoluminescence, and electrical properties of ZnO nanostructures synthesized by ice-bath assisted sonochemical method. J. Alloys Compd. 683, 399–411 (2016)
A.A. Othman, M.A. Osman, E.M.M. Ibrahim, M.A. Ali, A.G. Abd-Elrahim, Mn-doped ZnO nanocrystals synthesized by sonochemical method: structural, photoluminescence, and magnetic properties. Mater. Sci. Eng., B 219, 1–9 (2017)
M. Jothibas, C. Manoharan, S.J. Jeyakumar, P. Praveen, I.K. Punithavathy, J.P. Richard, Synthesis and enhanced photocatalytic property of Ni doped ZnS nanoparticles. Sol. Energy 159(2016), 434–443 (2018)
S. Kumar et al., Room temperature ferromagnetism in Ni doped ZnS nanoparticles. J. Alloys Compd. 554, 357–362 (2013)
H. Labiadh, B. Sellami, A. Khazri, W. Saidani, S. Khemais, Optical properties and toxicity of undoped and Mn-doped ZnS semiconductor nanoparticles synthesized through the aqueous route. Opt. Mater. (Amst) 64, 179–186 (2017)
G.J. Lee, S. Anandan, S.J. Masten, J.J. Wu, Sonochemical synthesis of hollow copper doped zinc sulfide nanostructures: optical and catalytic properties for visible light assisted photosplitting of water. Ind. Eng. Chem. Res. 53(21), 8766–8772 (2014)
B. Poornaprakash, U. Chalapathi, M. Reddeppa, S.H. Park, Effect of Gd doping on the structural, luminescence and magnetic properties of ZnS nanoparticles synthesized by the hydrothermal method. Superlattices Microstruct. 97, 104–109 (2016)
P. Scherrer, “Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen, in Kolloidchemie Ein Lehrbuch, ed. by R. Zsigmondy (Berlin, Springer, 1912), pp. 387–409
N. Daneu, S. Bernik, A. Renik, Inversion boundary induced grain growth in ZnO ceramics: from atomic-scale investigations to microstructural engineering. J. Phys: Conf. Ser. 326(1), 012003 (2011)
A.L. Allred, Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 17(3–4), 215–221 (1961)
A.I. Taub, C.L. Briant, Composition dependence of ductility in boron-doped, nickel-base L12 alloys. Acta Metall. 35(7), 1597–1603 (1987)
M.A. Osman, A.G. Abd-Elrahim, A.A. Othman, Identification of trapping and recombination levels, structure, morphology, photoluminescence and optical absorption behavior of alloyed ZnxCd1−xS quantum dots. J. Alloys Compd. 722, 344–357 (2017)
E.M. Hotze, T. Phenrat, G.V. Lowry, Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 39(6), 1909–1924 (2010)
H.C. Warad, S.C. Ghosh, B. Hemtanon, C. Thanachayanont, J. Dutta, Luminescent nanoparticles of Mn doped ZnS passivated with sodium hexametaphosphate. Sci. Technol. Adv. Mater. 6(3–4), 296–301 (2005)
M.A. Osman, A.G. Abd-Elrahim, A.A. Othman, Size-dependent structural phase transitions and their correlation with photoluminescence and optical absorption behavior of annealed Zn0.45Cd0.55S quantum dots. Mater. Charact. 144, 247–263 (2018)
E.J. Gonzalez, G.J. Piermarini, Handbook of Nanostructured Materials and Nanotechnology, vol. 1 (Academic Press, Cambridge, 2000)
Y. Kayanuma, Quantum-size effects of interacting electrons and holes in semiconductor microcrystals with spherical shape. Phys. Rev. B 38(14), 9797–9805 (1988)
H.S. Yoon et al., Properties of fluorine doped ZnO thin films deposited by magnetron sputtering. Sol. Energy Mater. Sol. Cells 92(11), 1366–1372 (2008)
O.I. Mićić et al., Size-dependent spectroscopy of InP quantum dots. J. Phys. Chem. B 101(25), 4904–4912 (1997)
N.K. Dutta, R.J. Nelson, The case for Auger recombination in In1−xGaxAsyP1−y. J. Appl. Phys. 53(1), 74–92 (1982)
D.-J. Jang, M.E. Flatté, C.H. Grein, J.T. Olesberg, T.C. Hasenberg, T.F. Boggess, Temperature dependence of Auger recombination in a multilayer narrow-band-gap superlattice. Phys. Rev. B 58(19), 13047 (1998)
V.I. Klimov, A.A. Mikhailovsky, D.W. McBranch, C.A. Leatherdale, M.G. Bawendi, Quantization of multiparticle Auger rates in semiconductor quantum dots. Science (80) 287(5455), 1011–1013 (2000)
Z.F. Liu, F.K. Shan, J.Y. Sohn, S.C. Kim, G.Y. Kim, Y.X. Li, Photoluminescence of ZnO: Ga thin films fabricated by pulsed laser deposition technique. J. Electroceramics 13(1–3), 183–187 (2004)
S. Muthukumaran, R. Gopalakrishnan, Structural, optical and photoluminescence studies of heavily Mn-doped ZnO nanoparticles annealed under Ar atmosphere. J. Mater. Sci.: Mater. Electron. 23(7), 1393–1401 (2012)
M.A. Osman, A.G. Abd-Elrahim, Excitation wavelength dependent photoluminescence emission behavior, UV induced photoluminescence enhancement and optical gap tuning of Zn0.45Cd0.55S nanoparticles for optoelectronic applications. Opt. Mater. (Amst) 77, 1–12 (2018)
P. Thomas, K.E. Abraham, Excitation wavelength dependent visible photoluminescence of CdO nanomorphotypes. J. Lumin. 158, 422–427 (2015)
T. Uematsu, S. Maenosono, Y. Yamaguchi, Photoinduced fluorescence enhancement in mono- And multilayer films of CdSe/ZnS quantum dots: dependence on intensity and wavelength of excitation light. J. Phys. Chem. B 109(18), 8613–8618 (2005)
O. Cherniavskaya, L. Chen, M.A. Islam, L. Brus, Photoionization of individual CdSe/CdS core/shell nanocrystals on silicon with 2-nm oxide depends on surface band bending. Nano Lett. 3(4), 497–501 (2003)
H.A. Le, L.T. Linh, S. Chin, J. Jurng, Photocatalytic degradation of methylene blue by a combination of TiO2 anatase and coconut shell activated carbon. Powder Technol. 225, 167–175 (2012)
M.-H. Baek, J.-W. Yoon, J.-S. Hong, J.-K. Suh, Application of TiO2 containing mesoporous spherical activated carbon in a fluidized bed photoreactor-adsorption and photocatalytic activity. Appl. Catal. A Gen. 450, 222–229 (2013)
Acknowledgements
The authors acknowledge the faculty of science Grant office at Assiut University for their financial support of this work. Also, many thanks to Prof. Dr. Abd El-Aziz A. Said for his kind assistance during this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Othman, A.A., Osman, M.A., Ali, M.A. et al. Sonochemically synthesized Ni-doped ZnS nanoparticles: structural, optical, and photocatalytic properties. J Mater Sci: Mater Electron 31, 1752–1767 (2020). https://doi.org/10.1007/s10854-019-02693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02693-z