Abstract
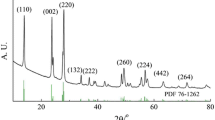
Effect of electrolytic anions on the charge storage mechanism and hence on the specific capacitance of Fe3O4 (magnetite) flexible thin film electrodes (FTFEs) has been studied. The synthesis of Fe3O4 on flexible stainless steel substrates has been carried out using successive ionic layer adsorption and reaction technique. 0.2 M FeCl3 and 0.1 M NaOH aqueous solutions were used as cationic and anionic sources respectively. X-ray diffraction pattern of the FTFE shows peaks at angles 2θ = 43.64° and 44.54° indicating the formation of orthorhombic Fe3O4 crystals of average size 10.25 nm. The scanning electron microscopic (SEM) image of FTFE shows irregularly stacked thin layers of interconnected nanograins. Surface wettability investigation concluded the hydrophilic nature of the prepared electrodes, as the measured contact angle was 10.5°. Thickness of the thin film observed by SEM cross section is ~267 nm. Cyclic voltammetric (CV) analyses substantiated that depending on the existence of electrolytic anion, the electrode stores charge either by physisorption-desorption or by redox reactions. FTFE shows maximum current hence the specific capacitance in KOH is more than SC in KCl. The observed maximum specific capacitance (SC) in 1 M aqueous solution of KCl was 206.53 F g−1 and that in 1 M aqueous solution of KOH was 542.21 F g−1 at 100 mV s−1. The galvanostatic charge–discharge analyses shows that the electrochemical performance of FTFE in KOH is better than that in KCl. The observed values of SCs at current density 1 mA cm−2 in KCl and KOH were 281.22 and 487.84 F g−1 respectively, which are nearly same as those given by the CV.



Similar content being viewed by others
References
B.E. Conway, Electrochemical Supercapacitors Scientific Fundamentals and Technological Applications (Kluwer-Plenum, New York, 1999)
R.C. Ambare, S.R. Bhardwaj, B.J. Lokhande, Non aqueous media dependent surface supercapacitive measurements of Co3O4 thin film electrodes prepared by spray pyrolysis. Int. J. Sci. Nat. 5(4), 663–668 (2014)
B.J. Lokhande, R.C. Ambare, R.S. Mane, S.R. Bharadwaj, Concentration-dependent electrochemical supercapacitive performance of Fe2O3. Curr. Appl. Phys. 13, 985–989 (2013)
R.M. Kore, R.S. Mane, M. Noushad, M.R. Khan, B.J. Lokhande, Nanomorphology dependent pseudocapacitive properties of NiO electrodes engineered through a controlled potentiodynamic electrodeposition process. RSC Adv. 6, 24478 (2016)
A. Barik, M. Mohapatra, α Fe2O3 nanomaterials: facet sensitive energy storage materials. Cryst. Eng. Comm. 17(47), 9203–9215 (2015)
R.S. ingole, B.J. Lokhande, Effect of pyrolysis temperature on structural, morphological and electrochemical properties of vanadium oxide thin films. Appl. Surf. Sci. 349, 887–896 (2015)
R.S. ingole, B.J. Lokhande, Spray pyrolyzed vanadium oxide thin films using different ingredients for redox supercapacitors. JMSE 27(2), 1363–1369 (2016)
P.R. Deshmukh, S.N. Pusawale, A.D. Jagadale, C.D. Lokhande, Supercapacitive performance of hydrous ruthenium oxide (RuO2·nH2O) thin films deposited by SILAR method. J. Mater. Sci. 7(3), 1546–1553 (2012)
P.M. Kulal, D.P. Dubal, C.D. Lokhande, V.J. Fulari, Chemical synthesis of Fe2O3 thin films for supercapacitor application. J. Alloys Compd. 509, 2567–2571 (2011)
J. Chen, K.L. Huang, S.Q. Liu, Hydrothermal preparation of octadecahedron Fe3O4 thin films for use in an electrochemical supercapacitor. Electrochim. Acta 55(1), 1–5 (2009)
A.V. Thakur, B.J. Lokhande, Effect of dip time on the electrochemical behavior of PPy-Cu(OH)2 hybrid electrodes synthesized using pyrrole and Cu(SO)4. ePolymer (2016). doi:10.1515/epoly-2016-0160
A.V. Thakur, B.J. Lokhande, Dip time dependent SILAR synthesis and electrochemical study of highly flexible PPy-Cu(OH)2 hybrid electrodes for supercapacitors. J. Solid State Electrochem. (2017). doi:10.1007/s10008-016-3502-2
C.D. Lokhande, D.P. Dubal, O.-S. Joo, Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 11, 255–270 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, A.V., Lokhande, B.J. Electrolytic anion affected charge storage mechanisms of Fe3O4 flexible thin film electrode in KCl and KOH: a comparative study by cyclic voltammetry and galvanostatic charge–discharge. J Mater Sci: Mater Electron 28, 11755–11761 (2017). https://doi.org/10.1007/s10854-017-6980-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6980-9