Abstract
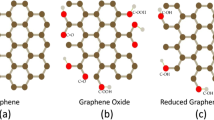
In this study, we obtained electrochemically reduced graphene oxide (ERGO) film via giving an appropriate negative potential to graphene oxide film on a glassy carbon electrode. The irreversible electrochemical reduction process and the enhancement of conductivity were ascribed to the decrease of oxygen groups of graphene oxide. With reduction potential becoming negatively, the resistance of ERGO declined obviously and the electrocatalytic ability of ERGO film were substantially improved toward probe molecules such as ascorbic acid and K3[Fe(CN)6]. Acid solution contributed to the reduction of graphene oxide and hydrogen ions participated in the electrochemical process. In NaOH solution, two cathodic peaks appeared at −1.40 V and −1.76 V, respectively. It is applicable to obtain ERGO film based on the favorable factors and details. This method is green and fast, and will not result in contamination of the reduced material. In addition, this approach shows promising application in electrochemical sensor field.









Similar content being viewed by others
References
A.K. Geim, Science 324, 1530–1534 (2009)
N.N. Klimov, S.Y. Jung, S.Z. Zhu, T. Li, A. Wright, S.D. Solares, D.B. Newell, N.B. Zhitenev, J.A. Stroscio, Science 336, 1557–1561 (2012)
Q.H. Wang, Z. Jin, K.K. Kim, A.J. Hilmer, G.L.C. Paulus, G.J. Shih, M.H. Ham, J.D. Sanchez-Yamagishi, K. Watanabe, T. Taniguchi, J. Kong, P. Jarillo-Herrero, M.S. Strano, Nat. Chem. 4, 724–732 (2012)
L. Liu, J. Park, D.A. Siegel, K.F. McCarty, K.W. Clark, W. Deng, L. Basile, J.C. Idrobo, A.P. Li, G. Gu, Science 343, 163–167 (2014)
R.K. Joshi, P. Carbone, F.C. Wang, V.G. Kravets, Y. Su, I.V. Grigorieva, H.A. Wu, A.K. Geim, R.R. Nair, Science 343, 752–754 (2014)
C.N.R. Rao, A.K. Sood, K.S. Subrahmanyam, A. Govindaraj, Angew. Chem. Int. Ed. 48, 7752–7777 (2009)
E. Bekyarova, M.E. Itkis, P. Ramesh, C. Berger, M. Sprinkle, W.A. Heer, R.C. Haddon, J. Am. Chem. Soc. 131, 1336–1337 (2009)
Q.H. Wang, M.C. Hersam, Nano Lett. 11, 589–593 (2011)
W. Chen, S. Chen, D.C. Qi, X.Y. Gao, A.T.S. Wee, J. Am. Chem. Soc. 129, 10418–10422 (2007)
G.H. Lu, L.E. Ocola, J.H. Chen, Nanotechnology 20, 445502–445511 (2009)
Y.G. Zhang, C.M. Liu, Y.L. Min, X.F. Qi, X.D. Ben, J. Mater. Sci. Mater. Electron 24, 3244–3248 (2013)
M. Ahmed, N. Kishi, R. Sugita, A. Fukaya, I. Khatri, J.B. Liang, S.M. Mominuzzaman, T. Soga, T. Jimbo, J. Mater. Sci. Mater. Electron 24, 2151–2155 (2013)
E. Casero, C. Alonso, L. Vazquez, M.D. Petit-Dominguez, A.M. Parra-Alfambra, M.D.L. Fuente, P. Merino, S. Alvarez-Garcia, A.D. Andrs, F. Pariente, E. Lorenzo, Electroanalysis 25, 154–165 (2013)
S. Patchkovskii, J.S. Tse, S.N. Yurchenko, L. Zhechkov, T. Heine, G. Seifert, PNAS 102, 10439–10444 (2005)
M. Keidar, A. Shashurin, O. Volotskova, Y. Raitses, I.I. Beilis, Phys. Plasmas 17, 057101–057109 (2010)
D.M. Sun, W.N. Hu, W. Ma, J. Anal. Chem. 66, 310–316 (2011)
C.S. Shan, H.F. Yang, D.X. Han, Q.X. Zhang, A. Ivaska, L. Niu, Biosens. Bioelectron. 25, 1504–1508 (2010)
Y. Wang, Y.M. Li, L.H. Tang, J. Lu, J.H. L. Electrochem. Commun. 11, 889–892 (2009)
Z.J. Wang, X.Z. Zhou, J. Zhang, F. Boey, H. Zhang, J. Phys. Chem. C 113, 14071–14075 (2009)
A.B. Kharitonov, L. Alfonta, E. Katz, I. Willner, J. Electroanal. Chem. 487, 133–141 (2000)
M. Zhou, Y.L. Wang, Y.M. Zhai, J.F. Zhai, W. Ren, F. Wang, S.J. Dong, Chem. Eur. J. 15, 6116–6120 (2009)
M. Pumera, Chem. Rec. 9, 211–223 (2009)
M. Pumera, R. Scipioni, H. Iwai, T. Ohno, Y. Miyahara, M. Boero, Chem. Eur. J. 15, 10851–10856 (2009)
H.L. Guo, X.F. Wang, Q.Y. Qian, F.B. Wang, X.H. Xia, ACS Nano 3, 2653–2659 (2009)
Acknowledgments
This research is funded by Anhui Province Universities Provincial Natural Science Research Foundation for Key Project (No. KJ2011A255), China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, G., Wang, Y. & Sun, D. Investigation of conductivity and catalytic ability at an electrochemically reduced graphene oxide film modified electrode. J Mater Sci: Mater Electron 26, 943–949 (2015). https://doi.org/10.1007/s10854-014-2486-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2486-x