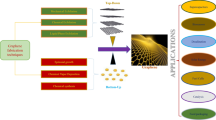
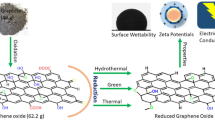
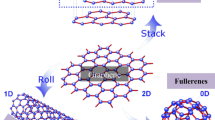
Abstract
As nanotechnology floods application areas like medicine, electronics, water remediation, space and textiles, just to name a few, some nanomaterials remain in the spotlight due to their fantastic properties and their incredible potential. Such is the case of the 2D, transparent, flexible, strong, carbon-based nanomaterial called graphene. Graphene consists of sp2 hybridized carbon arranged in a flat network packed in a honey-comb pattern, having thus mono-atomic thickness. Its isolation in 2004 opened the door to numerous investigations and its research is funded each year by governments, industries and academia worldwide. Due to its non-hydrophilic nature, some applications represent a challenge (particularly biological and medical applications), thus an oxygen/hydrogen-functionalized hydrophilic version of it has lately gained popularity, its name is graphene oxide. This document aims to review the synthesis methods of graphene, graphene oxide and reduced graphene oxide. A revision of the most important top-down and bottom-up methods is presented, focusing on chemical vapor deposition for the growth of graphene and the wet-chemical methods for the synthesis of graphene oxide and the reduction techniques available for reduced graphene oxide. We conclude by analyzing the current situation of graphene and graphene oxide production and the challenges that need to be tackled in order to meet the short-term demand of these nanomaterials for the promised applications.

(Source: xyz coordinates of the structures CSIRO Data Access Portal)







Similar content being viewed by others
References
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191. https://doi.org/10.1038/nmat1849
Bhuyan MSA, Uddin MN, Islam MM, Bipasha FA, Hossain SS (2016) Synthesis of graphene. NInt Nano Lett 6:65–83. https://doi.org/10.1007/s40089-015-0176-1
Huang PY et al (2011) Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature 469:389–392. https://doi.org/10.1038/nature09718
Malard LM, Pimenta MA, Dresselhaus G, Dresselhaus MS (2009) Raman spectroscopy in graphene. Phys Rep 473:51–87. https://doi.org/10.1016/j.physrep.2009.02.003
Ferralis N (2010) Probing mechanical properties of graphene with Raman spectroscopy. J Mater Sci 45:5135–5149. https://doi.org/10.1007/s10853-010-4673-3
Yi M, Shen Z (2015) A review on mechanical exfoliation for the scalable production of graphene. J Mater Chem 3:11700–11715. https://doi.org/10.1039/C5TA00252D
Lee HC, Liu W-W, Chai S-P, Mohamed AR, Aziz A, Khe C-S, Hidayaha NMS, Hashima U (2017) Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv 7:15644–15693. https://doi.org/10.1039/C7RA00392G
Wu Y, Wang S, Komvopoulos K (2020) A review of graphene synthesis by indirect and direct deposition methods. J Mater Res 35:76–89. https://doi.org/10.1557/jmr.2019.377
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22:3906–3924. https://doi.org/10.1002/adma.201001068
Adetayo A, Runsewe D (2019) Synthesis and fabrication of graphene and graphene oxide: a review. Open J Compos Mater 9:207–229. https://doi.org/10.4236/ojcm.2019.92012
Agarwal V, Zetterlund PB (2021) Strategies for reduction of graphene oxide—a comprehensive review. Chem Eng J 405:127018. https://doi.org/10.1016/j.cej.2020.127018
Novoselov KS, Geim A, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669. https://doi.org/10.1126/science.1102896
Gumfekar SP (2018) Graphene-based materials for clean energy applications. In: Bhanvase BA, Pawade VB, Dhoble SJ, Sonawane SH, Ashokkumar M (eds) Nanomaterials for Green Energy. Elsevier, Amsterdam, pp 351–383. https://doi.org/10.1016/B978-0-12-813731-4.00011-4
Pirzado AA, Le Normand F, Romero T, Paszkiewicz S, Papaefthimiou V, Ihiawakrim D, Janowska I (2019) Few-layer graphene from mechanical exfoliation of graphite-based materials: structure-dependent characteristics. Chem Eng 3:37. https://doi.org/10.3390/chemengineering3020037
Huang Y, Sutter E, Shi NN, Zheng J, Yang T, Englund D, Gao HJ, Sutter P (2015) Reliable exfoliation of large-area high-quality flakes of graphene and other two-dimensional materials. ACS Nano 9:10612–10620. https://doi.org/10.1021/acsnano.5b04258
Hernandez Y et al (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 3:563–568. https://doi.org/10.1038/nnano.2008.215
Bourlinos AB, Georgakilas V, Zboril R, Steriotis TA, Stubos AK (2009) Liquid-phase exfoliation of graphite towards solubilized graphenes. Small 5:1841–1845. https://doi.org/10.1002/smll.200900242
Zhao W, Fang M, Wu F, Wu H, Wang L, Chen G (2010) Preparation of graphene by exfoliation of graphite using wet ball milling. J Mater Chem 20:5817–5819. https://doi.org/10.1039/c0jm01354d
Lv Y, Yu L, Jiang C, Chenb S, Nie Z (2014) Synthesis of graphene nanosheet powder with layer number control via a soluble salt-assisted route. RSC Adv 4:13350–13354. https://doi.org/10.1039/c3ra45060k
Lin T, Tang Y, Wang Y, Bi H, Liu Z, Huang F, Xiec X, Jiang M (2013) Scotch-tape-like exfoliation of graphite assisted with elemental sulfur and graphene–sulfur composites for high-performance lithium-sulfur batteries. Energy Environ Sci 6:1283–1290. https://doi.org/10.1039/c3ee24324a
Carozo V, Almeida CM, Ferreira EHM, Cançado LG, Achete CA, Jorio A (2011) Raman signature of graphene superlattices. Nano Lett 11:4527–4534. https://doi.org/10.1021/nl201370m
Wang J, Mu X, Wang L, Sun M (2019) Properties and applications of new superlattice: twisted bilayer graphene. Mater Today Phys. https://doi.org/10.1016/j.mtphys.2019.100099
Raji M, Zari N, Bouhfid R (2019) Chemical preparation and functionalization techniques of graphene and graphene oxide. In: Raji M, Zari N, El Kacem Qaiss A, Bouhfid R (eds) Functionalized graphene nanocomposites and their derivatives. Elsevier, Amsterdam, pp 1–20. https://doi.org/10.1016/B978-0-12-814548-7.00001-5
Seekaew Y, Arayawut O, Timsorn K, Wongchoosuk C (2019) Synthesis, characterization, and applications of graphene and derivatives. In: Yaragalla S, Mishra RK, Thomas S, Kalarikkal N, Maria HJ (eds) Carbon-based nanofillers and their rubber Nanocomposites. Amsterdam, Elsevier, pp 259–283. https://doi.org/10.1016/B978-0-12-813248-7.00009-2
Viculis LM, Mack JJ, Kaner RB (2003) A chemical route to carbon nanoscrolls. Science 299:1361. https://doi.org/10.1126/science.1078842
Parvez K, Yang S, Feng X, Müllen K (2015) Exfoliation of graphene via wet chemical routes. Synth Met 210:123–132. https://doi.org/10.1016/j.synthmet.2015.07.014
Xia ZY, Pezzini S, Treossi E, Giambastiani G, Corticelli F, Morandi V, Zanelli A, Bellani V, Palermo V (2013) The exfoliation of graphene in liquids by electrochemical, chemical, and sonication-assisted techniques: a nanoscale study. Adv Funct Mater 23:4684–4693. https://doi.org/10.1002/adfm.201203686
Liu N, Luo F, Wu H, Liu Y, Zhang C, Chen J (2008) One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv Funct Mater 18:1518–1525. https://doi.org/10.1002/adfm.200700797
Hass J, De Heer WA, Conrad EH (2008) The growth and morphology of epitaxial multilayer graphene. J Phys Cond Matt 20:323202. https://doi.org/10.1088/0953-8984/20/32/323202
De Heer WA et al (2007) Epitaxial graphene. Solid State Commun 143:92–100. https://doi.org/10.1016/j.ssc.2007.04.023
Berger C, Song Z, Li T, Li X, Ogbazghi AY, Feng R, Dai Z, Marchenkov AN, Conrad EH, First PN, De Heer WA (2004) Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J Phys Chem B 108:19912–19916. https://doi.org/10.1021/jp040650f
Rollings E, Gweon GH, Zhou SY, Mun BS, McChesney JL, Hussain BS, Fedorov AV, First PN, De Heer WA, Lanzara A (2006) Synthesis and characterization of atomically thin graphite films on a silicon carbide substrate. J Phys Chem Solids 67:2172–2177. https://doi.org/10.1016/j.jpcs.2006.05.010
Berger C et al (2006) Electronic confinement and coherence in patterned epitaxial graphene. Science 312:1191–1196. https://doi.org/10.1126/science.1125925
Wu X, Li X, Song Z, Berger C, De Heer WA (2007) Weak antilocalization in epitaxial graphene: evidence for chiral electrons. Phys. Rev. Lett. 98:136801-1–136801-4. https://doi.org/10.1103/PhysRevLett.98.136801
Virojanadara C, Yakimova R, Zakharov AA, Johansson LI (2010) Large homogeneous mono-/bi-layer graphene on 6H–SiC (0 0 0 1) and buffer layer elimination. J Phys D Appl Phys 43:374010. https://doi.org/10.1088/0022-3727/43/37/374010
Emtsev KV et al (2009) Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat Mater 8:203–207. https://doi.org/10.1038/nmat2382
De Heer WA, Berger C, Ruan M, Sprinkle M, Li X, Hu Y, Zhang B, Hankinson J, Conrad E (2011) Large area and structured epitaxial graphene produced by confinement controlled sublimation of silicon carbide. PNAS 108:16900–16905. https://doi.org/10.1073/pnas.1105113108
Tromp RM, Hannon JB (2009) Thermodynamics and kinetics of graphene growth on SiC (0001). Phys Rev Lett 102:106104. https://doi.org/10.1103/PhysRevLett.102.106104
Miao C (2011) CVD Synthesis of Graphene and Graphene Bipolar Junction Transistor. Thesis, University of California, Los Angeles. https://www.proquest.com/openview/44049b07d87df250e66566241845e4da/1?pq-origsite=gscholar&cbl=18750 (Accessed 2021-11-15).
Chen Q, Zhou M, Zhang Z, Tang T, Wang T (2017) Preparation of TiO2 nanotubes/reduced graphene oxide binary nanocomposites enhanced photocatalytic properties. J Mater Sci Mater Electron 28:9416–9422. https://doi.org/10.1007/s10854-017-6683-2
Ma LP, Ren W, Cheng HM (2019) Transfer methods of graphene from metal substrates: a review. Small Methods 3(7):1900049. https://doi.org/10.1002/smtd.201900049
Xu S, Zhang L, Wang B, Ruoff RS (2021) Chemical vapor deposition of graphene on thin-metal films. Cell Rep 2:100372-1–100372-35. https://doi.org/10.1016/j.xcrp.2021.100372
Martin PM (2009). In: Martin PM (ed) Handbook of deposition technologies for films and coatings: science, applications and technology. Elsevier, Amsterdam
Somani PR, Somani SP, Umeno M (2006) Planer nano-graphenes from camphor by CVD. Chem Phys Lett 430:56–59. https://doi.org/10.1016/j.cplett.2006.06.081
Yu Q, Lian J, Siriponglert S, Li H, Chen YP, Pei SS (2008) Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 93:113103. https://doi.org/10.1063/1.2982585
De Arco LG, Zhang Y, Kumar A, Zhou C (2009) Synthesis, transfer, and devices of single-and few-layer graphene by chemical vapor deposition. TNANO 8:135–138. https://doi.org/10.1109/TNANO.2009.2013620
Reina A, Jia X, Ho J, Nezich D, Son H, Bulovic V, Dresselhaus MS, Kong J (2009) Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett 9:30–35. https://doi.org/10.1021/nl801827v
Kim KS et al (2009) Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457:706–710. https://doi.org/10.1038/nature07719
Li X et al (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324:1312–1314. https://doi.org/10.1126/science.1171245
Li X, Cai W, Colombo L, Ruoff RS (2009) Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett 9:4268–4272. https://doi.org/10.1021/nl902515k
Kang BJ, Mun JH, Hwang CY, Cho BJ (2009) Monolayer graphene growth on sputtered thin film platinum. J Appl Phys 106:104309-1–104309-6. https://doi.org/10.1063/1.3254193
Coraux J, Plasa TN, Busse C, Michely T (2008) Structure of epitaxial graphene on Ir (111). New J Phys 10:043033-1–043033-16. https://doi.org/10.1021/nl0728874
Varykhalov A, Rader O (2009) Graphene grown on Co (0001) films and islands: Electronic structure and its precise magnetization dependence. Phys Rev B 80:035437-1–035437-6. https://doi.org/10.1103/PhysRevB.80.035437
Kwon SY, Ciobanu CV, Petrova V, Shenoy VB, Bareno J, Gambin V, Petrov I, Kodambaka S (2009) Growth of semiconducting graphene on palladium. Nano Lett. 9:3985–3990. https://doi.org/10.1021/nl902140j
Nandamuri G, Roumimov S, Solanki R (2010) Chemical vapor deposition of graphene films. Nanotechnology 21:145604. https://doi.org/10.1088/0957-4484/21/14/145604
Kim J, Seo, J, Jung HK, Kim SH, Lee HW (2012) The effect of various parameters for few-layered graphene synthesis using methane and acetylene. J Ceram Process Res 13:S42–S46. https://doi.org/10.36410/jcpr.2012.13.42
Qi M, Ren Z, Jiao Y, Zhou Y, Xu X, Li W, Zheng X, Bai J (2013) Hydrogen kinetics on scalable graphene growth by atmospheric pressure chemical vapor deposition with acetylene. J Phys Chem C 117:14348–14353. https://doi.org/10.1021/jp403410b
Mueller NS, Morfa AJ, Abou-Ras D, Oddone V, Ciuk T, Giersig M (2014) Growing graphene on polycrystalline copper foils by ultra-high vacuum chemical vapor deposition. Carbon 78:347–355. https://doi.org/10.1016/j.carbon.2014.07.011
Yang M, Sasaki S, Ohnishi M, Suzuki K, Miura H (2016) Electronic properties and strain sensitivity of CVD-grown graphene with acetylene. Jpn J Appl Phys 55:04EP05. https://doi.org/10.7567/JJAP.55.04EP05
Stadermann M et al (2009) Mechanism and kinetics of growth termination in controlled chemical vapor deposition growth of multiwall carbon nanotube arrays. Nano Lett 9:738–744. https://doi.org/10.1021/nl803277g
De Parga AV, Calleja F, Borca BMCG, Passeggi MCG Jr, Hinarejos JJ, Guinea F, Miranda R (2008) Periodically rippled graphene: growth and spatially resolved electronic structure. Phys Rev Lett 100:056807–056807-4. https://doi.org/10.1103/PhysRevLett.100.056807
Celebi K, Cole MT, Teo KB, Park HG (2011) Observations of early stage graphene growth on copper. Electrochem Solid-State Lett 15:K1–K4. https://doi.org/10.1149/2.005201esl
Addou R, Dahal A, Sutter P, Batzill M (2012) Monolayer graphene growth on Ni (111) by low temperature chemical vapor deposition. Appl Phys Lett 100:021601. https://doi.org/10.1063/1.3675481
Li Z, Wu P, Wang C, Fan X, Zhang W, Zhai X, Zeng C, Li C, Yang J, Hou J (2011) Low-temperature growth of graphene by chemical vapor deposition using solid and liquid carbon sources. ACS Nano 5:3385–3390. https://doi.org/10.1021/nn200854p
Campos-Delgado J, Botello-Méndez AR, Algara-Siller G, Hackens B, Pardoen T, Kaiser U, Dresselhaus MS, Charlier JC, Raskin JP (2013) CVD synthesis of mono-and few-layer graphene using alcohols at low hydrogen concentration and atmospheric pressure. Chem. Phys. Lett 584:142–146. https://doi.org/10.1016/j.cplett.2013.08.031
Miyata Y, Kamon K, Ohashi K, Kitaura R, Yoshimura M, Shinohara H (2010) A simple alcohol-chemical vapor deposition synthesis of single-layer graphenes using flash cooling. Appl Phys Lett 96:263105–263105-3. https://doi.org/10.1063/1.3458797
Srivastava A, Galande C, Ci L, Song L, Rai C, Jariwala D, Kelly KF, Ajayan PM (2010) Novel liquid precursor-based facile synthesis of large-area continuous, single, and few-layer graphene films. Chem Mater 22:3457–3461. https://doi.org/10.1021/cm101027c
Guermoune A, Chari T, Popescu F, Sabri SS, Guillemette J, Skulason HS, Szkopek T, Siaj M (2011) Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 49:4204–4210. https://doi.org/10.1016/j.carbon.2011.05.054
Gadipelli S, Calizo I, Ford J, Cheng G, Walker ARH, Yildirim T (2011) A highly practical route for large-area, single layer graphene from liquid carbon sources such as benzene and methanol. J Mat Chem 21:16057–16065. https://doi.org/10.1039/C1JM12938D
Maarof S, Ali AA, Hashim AM (2019) Synthesis of large-area single-layer graphene using refined cooking palm oil on copper substrate by spray injector-assisted CVD. Nanoscale Res Lett 14:1–8. https://doi.org/10.1186/s11671-019-2976-0
Li Z, Gordon RG, Pallem V, Li H, Shenai DV (2010) Direct-liquid-injection chemical vapor deposition of nickel nitride films and their reduction to nickel films. Chem Mater 22:3060–3066. https://doi.org/10.1021/cm903636j
Intaro T et al (2020) Characterization of graphene grown by direct-liquid-injection chemical vapor deposition with cyclohexane precursor in N2 ambient. Diam Relat Mat 104:107717. https://doi.org/10.1016/j.diamond.2020.107717
Sun Z, Yan Z, Yao J, Beitler E, Zhu Y, Tour JM (2010) Growth of graphene from solid carbon sources. Nature 468:549–552. https://doi.org/10.1038/nature09579
Ahmed M, Kishi N, Sugita R, Fukaya A, Khatri I, Liang J, Mominuzzaman SM, Soga T, Jimbo T (2013) Graphene synthesis by thermal chemical vapor deposition using solid precursor. J Mater Sci Mater Electron 24:2151–2155. https://doi.org/10.1007/s10854-013-1073-x
Kondrashov II, Rybinm MG, Obraztsova EA, Obraztsova ED (2019) Controlled graphene synthesis from solid carbon sources. Phys Status Solidi B 256:1800688. https://doi.org/10.1002/pssb.201800688
Ruan G, Sun Z, Peng Z, Tour JM (2011) Growth of graphene from food, insects, and waste. ACS Nano 5:7601–7607. https://doi.org/10.1021/nn202625c
Sharma S, Kalita G, Hirano R, Shinde SM, Papon R, Ohtani H, Tanemura M (2014) Synthesis of graphene crystals from solid waste plastic by chemical vapor deposition. Carbon 72:66–73. https://doi.org/10.1016/j.carbon.2014.01.051
Weatherup RS, Bayer BC, Blume R, Ducati C, Baehtz C, Schlögl R, Hofmann S (2011) In situ characterization of alloy catalysts for low-temperature graphene growth. Nano Lett 11:4154–4160. https://doi.org/10.1021/nl202036y
Chen S, Cai W, Piner RD, Suk JW, Wu Y, Ren Y, Kang J, Ruoff RS (2011) Synthesis and characterization of large-area graphene and graphite films on commercial Cu–Ni alloy foils. Nanolett 11:3519–3525. https://doi.org/10.1021/nl201699j
Huang M et al (2018) Highly oriented monolayer graphene grown on a Cu/Ni (111) alloy foil. ACS Nano 12:6117–6127. https://doi.org/10.1021/acsnano.8b02444
Li X, Magnuson CW, Venugopal A, Tromp RM, Hannon JB, Vogel EM, Colombo L, Ruoff RS (2011) Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J Am Chem Soc 133:2816–2819. https://doi.org/10.1021/ja109793s
Luo B et al (2017) Sputtering an exterior metal coating on copper enclosure for large-scale growth of single-crystalline graphene. 2D Mater 4:5017. https://doi.org/10.1088/2053-1583/aa85d5
Hao Y et al (2013) The role of surface oxygen in the growth of large single-crystal graphene on copper. Science 342:720–723. https://doi.org/10.1126/science.1243879
Liang T, Luan C, Chen H, Xu M (2017) Exploring oxygen in graphene chemical vapor deposition synthesis. Nanoscale 9:3719–3735. https://doi.org/10.1039/C7NR00188F
Cao QJ, Shi BY, Dou WD, Tang JX, Mao HY (2018) Background pressure does matter for the growth of graphene single crystal on copper foil: Key roles of oxygen partial pressure. Carbon 138:458–464. https://doi.org/10.1016/j.carbon.2018.07.072
Hesjedal T (2011) Continuous roll-to-roll growth of graphene films by chemical vapor deposition. Appl Phys Lett 98:133106–133106-3. https://doi.org/10.1063/1.3573866
Kobayashi T et al (2013) Production of a 100-m-long high-quality graphene transparent conductive film by roll-to-roll chemical vapor deposition and transfer process. Appl Phys Lett 102:023112. https://doi.org/10.1063/1.4776707
Polsen ES, McNerny DQ, Viswanath B, Pattinson SW, Hart AJ (2015) High-speed roll-to-roll manufacturing of graphene using a concentric tube CVD reactor. Sci Rep 5:1–12. https://doi.org/10.1038/srep10257
Jalili M, Ghanbari H, Bellah SM, Malekfa R (2019) High-quality liquid phase-pulsed laser ablation graphene synthesis by flexible graphite exfoliation. J Mater Res Technol 35:292–299. https://doi.org/10.1016/j.jmst.2018.09.048
Ye R, James DK, Tour JM (2019) Laser-induced graphene: from discovery to translation. Adv Mater 31:1803621. https://doi.org/10.1002/adma.201803621
Stanford MG, Zhang C, Fowlkes JD, Hoffman A, Ivanov IN, Rack PD, Tour JM (2020) High-resolution laser-induced graphene flexible electronics beyond the visible limit. ACS Appl Mater Interfaces 12:10902–10907. https://doi.org/10.1021/acsami.0c01377
Beckham JL, Li JT, Stanford MG, Chen W, McHugh EA, Advincula PA, Wyss KM, Chyan Y, Boldman WL, Rack PD, Tour JM (2021) High-Resolution laser-induced graphene from photoresist. ACS Nano 15:8976–8983. https://doi.org/10.1021/acsnano.1c01843
Lin J, Peng Z, Liu Y, Ruiz-Zepeda F, Ye R, Samuel ELG, Yacaman MJ, Yakobson BI, Tour JM (2014) Laser-induced porous graphene films from commercial polymers. Nat Commun 5:5714. https://doi.org/10.1038/ncomms6714
Ye R, James DK, Tour JM (2018) Laser-induced graphene. Acc Chem Res 51:1609–1620. https://doi.org/10.1021/acs.accounts.8b00084
Wang L, Wang Z, Bakhtiyari AN, Zheng H (2020) A comparative study of laser-induced graphene by CO2 infrared laser and 355 nm ultraviolet (UV) laser. Micromachines 11:1094. https://doi.org/10.3390/mi11121094
Kulyk B, Silva BFR, Carvalho AF, Silvestre S, Fernandes AJS, Martins R, Fortunato E, Costa FM (2021) Laser-induced graphene from paper for mechanical sensing. ACS Appl Mater Interfaces 13:10210–10221. https://doi.org/10.1021/acsami.0c20270
Qian M, Zhou YS, Gao Y, Park JB, Feng T, Huang SM, Sun Z, Jiang L, Lu YF (2011) Formation of graphene sheets through laser exfoliation of highly ordered pyrolytic graphite. Appl Phys. Lett. 98:173. https://doi.org/10.1063/1.3584021
Kiran GR, Chandu B, Acharyya SW, Rao VS, Srikanth VVSS (2017) One-step synthesis of bulk quantities of graphene from graphite by femtosecond laser ablation under ambient conditions. Philos Mag Lett 97:229–234. https://doi.org/10.1080/09500839.2017.1320437
Hameed R, Khashanb KS, Sulaiman GM (2020) Preparation and characterization of graphene sheet prepared by laser ablation in liquid. Mater Today Proc 20:535–539. https://doi.org/10.1016/j.matpr.2019.09.185
Russo P, Hu A, Compagnini G, Duley WW, Zhoua NY (2014) Femtosecond laser ablation of highly orientedpyrolytic graphite: a green route for large-scale production of porous graphene and graphene quantum dots. Nanoscale 6:2381–2389. https://doi.org/10.1039/C3NR05572H
Sadeghi H, Solati E, Dorranian D (2019) Producing graphene nanosheets by pulsed laser ablation: effects of liquid environment. Laser Appl 31:042003. https://doi.org/10.2351/1.5109424
Huang Y, Sepioni M, Whitehead D, Liu Z, Guo W, Zhong X, Gu H, Li L (2020) Rapid growth of large area graphene onglass from olive oil by laser irradiation. Nanotechnology 31:245601. https://doi.org/10.1088/1361-6528/ab7ef6
Luong DX et al (2020) Gram-scale bottom-up flash graphene synthesis. Nature 577:647–651. https://doi.org/10.1038/s41586-020-1938-0
Stanford MG et al (2020) Flash graphene morphologies. ACS Nano 14:13691–13699. https://doi.org/10.1021/acsnano.0c05900
Algozeeb WA, Savas PE, Luong DX, Chen W, Kittrell C, Bhat M, Shahsavari R, Tour JM (2020) Flash graphene from plastic waste. ACS Nano 14:15595–15604. https://doi.org/10.1021/acsnano.0c06328
Advincula PA, Luong DX, Chen W, Raghuraman S, Shahsavari R, Tour JM (2021) Flash graphene from rubber waste. Carbon 178:649–656. https://doi.org/10.1016/j.carbon.2021.03.020
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Schniepp HC, Li J-L, McAllister MJ, Sai H, Herrera-Alonso M, Adamson DH, Prudhomme RK, Car R, Saville DA, Aksay IA (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 110:8535–8539. https://doi.org/10.1021/jp060936f
Poh HL, Šaněk F, Ambrosi A, Zhao G, Sofer Z, Pumera M (2012) Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 4:3515–3522. https://doi.org/10.1039/C2NR30490B
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339. https://doi.org/10.1021/ja01539a017
Chen J, Yao B, Li C, Shi G (2013) An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64:225–229. https://doi.org/10.1016/j.carbon.2013.07.055
Akhavan O, Ghaderi E (2009) Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J Phys Chem C 113:20214–20220. https://doi.org/10.1021/jp906325q
Perera SD, Mariano RG, Vu K, Nour N, Seitz O, Chabal Y, Balkus KJ Jr (2012) Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal 2:949–956. https://doi.org/10.1021/cs200621c
Lingappan N, Gal Y-S, Lim KT (2013) Synthesis of reduced graphene oxide/polypyrrole conductive composites. Mol Cryst Liq Cryst 585:60–66. https://doi.org/10.1080/15421406.2013.849510
Sim LC, Leong KH, Ibrahim S, Saravanan P (2014) Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electrons mobility and visible-light driven photocatalytic performance. J Mater Chem A 2:5315–5322. https://doi.org/10.1039/C3TA14857B
Yu H, Zhang B, Bulin C, Li R, Xing R (2016) High-efficient synthesis of graphene oxide based on improved hummers method. Sci Rep 6:1–7. https://doi.org/10.1038/srep36143
Zaaba NI, Foo KL, Hashim U, Tan SJ, Liu W, Voon CH (2017) Synthesis of graphene oxide using modified hummers method: solvent influence. Proc Eng 184:469–477. https://doi.org/10.1016/j.proeng.2017.04.118
Alam SN, Sharma N, Kumar L (2017) Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 6:1–18. https://doi.org/10.4236/graphene.2017.61001
Liu F, Wang C, Sui X, Riaz MA, Xu M, Wei L, Chen Y (2019) Synthesis of graphene materials by electrochemical exfoliation: recent progress and future potential. Carb Energy 1:173–199. https://doi.org/10.1002/cey2.14
Kakaei K, Hasanpour K (2014) Synthesis of graphene oxide nanosheets by electrochemical exfoliation of graphite in cetyltrimethylammonium bromide and its application for oxygen reduction. J Mater Chem A 2:15428–15436. https://doi.org/10.1039/C4TA03026E
Md Disa N, Abu Bakar S, Alfarisa SUHUFA, Mohamed AZMI, Md Isa I, Kamari AZLAN et al (2015) The synthesis of graphene oxide via electrochemical exfoliation method. Adv. Mater. Res. 1109:55–59. https://doi.org/10.4028/www.scientific.net/AMR.1109.55
De Silva KKH, Huang H-H, Joshi RK, Yoshimura M (2017) Chemical reduction of graphene oxide using green reductants. Carbon 119:190–199. https://doi.org/10.1016/j.carbon.2017.04.025
Toh SY, Loh KS, Kamarudin SK, Daud WRW (2014) Graphene production via electrochemical reduction of graphene oxide: synthesis and characterisation. Chem Eng J 251:422–434. https://doi.org/10.1016/j.cej.2014.04.004
Sim LC, Leong KH, Saravanan P, Ibrahim S (2015) Rapid thermal reduced graphene oxide/Pt–TiO2 nanotube arrays for enhanced visible-light-driven photocatalytic reduction of CO2. App Surf Sci 358:122–129. https://doi.org/10.1016/j.apsusc.2015.08.065
Sengupta I, Chakraborty S, Talukdar M, Pal SK, Chakraborty S (2018) Thermal reduction of graphene oxide: how temperature influences purity. J Mater Res 33:4113–4122. https://doi.org/10.1557/jmr.2018.338
Chen W, Yan L, Bangal PR (2010) Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 48:1146–1152. https://doi.org/10.1016/j.carbon.2009.11.037
Zhu Y, Murali S, Stoller MD, Velamakanni A, Piner RD, Ruoff RS (2010) Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 48:2118–2122. https://doi.org/10.1016/j.carbon.2010.02.001
Zheng X, Peng Y, Yang Y, Chen J, Tian H, Cui X, Zheng W (2016) Hydrothermal reduction of graphene oxide; effect on surface-enhanced Raman scattering. J Raman Spectrosc 48:97–103. https://doi.org/10.1002/jrs.4998
Huang HH, De Silva KKH, Kumara GRA, Yoshimura M (2018) Structural evolution of hydrothermally derived reduced graphene oxide. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-25194-1
Huang HH, Joshi RK, De Silva KKH, Badam R, Yoshimura M (2018) Fabrication of reduced graphene oxide membranes for desalination. J Memb Sci 572:12–19. https://doi.org/10.1016/j.memsci.2018.10.085
Zhou Y, Bao Q, Tang LAL, Zhong Y, Loh KP (2009) Hydrothermal dehydration for the “Green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem Mater 21:2950–2956. https://doi.org/10.1021/cm9006603
Mei X, Meng X, Wu F (2015) Hydrothermal method for the production of reduced graphene oxide. Phys E Low-Dimens Syst Nanostruct 68:81–86. https://doi.org/10.1016/j.physe.2014.12.011
Rambabu Y, Kumar U, Singhal N, Kaushal M, Jaiswal M, Hain SL, Roy SC (2019) Photocatalytic reduction of carbon dioxide using graphene oxide wrapped TiO2 nanotubes. App Surf Sci 485:48–55. https://doi.org/10.1016/j.apsusc.2019.04.041
Gallegos-Pérez WR, Reynosa-Martínez AC, Soto-Ortiz C, Angélica Álvarez-Lemus M, Barroso-Flores J, García Montalvo V, López-Honorato E (2020) Effect of UV radiation on the structure of graphene oxide in water and its impact on cytotoxicity and As(III) adsorption. Chemosphere 249:126160. https://doi.org/10.1016/j.chemosphere.2020.126160
Guardia L, Villar-Rodil S, Paredes JI, Rozada R, Martínez-Alonso A, Tascón JMD (2012) UV light exposure of aqueous graphene oxide suspensions to promote their direct reduction, formation of graphene–metal nanoparticle hybrids and dye degradation. Carbon 50:1014–1024. https://doi.org/10.1016/j.carbon.2011.10.005
Mohandoss M, Gupta SS, Nelleri A, Pradeep T, Maliyekkal SM (2017) Solar mediated reduction of graphene oxide. RSC Adv 7:957–963. https://doi.org/10.1039/c6ra24696f
Hou WC, Chowdhury I, Goodwin DG, Henderson WM, Fairbrother DH, Bouchard D, Zepp RG (2015) Photochemical transformation of graphene oxide in sunlight. Environ Sci Technol 49:3435–3443. https://doi.org/10.1021/nn101390x
Kauppila J, Kunnas P, Damlin P, Viinikanoja A, Kvarnström C (2013) Electrochemical reduction of graphene oxide films in aqueous and organic solutions. Electrochim Acta 89:84–89. https://doi.org/10.1016/j.electacta.2012.10.153
Jiang Y, Lu Y, Li F, Wu T, Niu L, Chen W (2012) Facile electrochemical codeposition of “clean” graphene–Pd nanocomposite as an anode catalyst for formic acid electrooxidation. Electrochem Commun 19:21–24. https://doi.org/10.1016/j.elecom.2012.02.035
Guo H-L, Wang X-F, Qian Q-Y, Wang F-B, Xia X-H (2009) A Green Approach to the synthesis of graphene nanosheets. ACS Nano 3:2653–2659. https://doi.org/10.1021/nn900227d
Yang J, Deng S, Lei J, Ju H, Gunasekaran S (2011) Electrochemical synthesis of reduced graphene sheet–AuPd alloy nanoparticle composites for enzymatic biosensing. Biosens Bioelectron 29:159–166. https://doi.org/10.1016/j.bios.2011.08.011
Peng X-Y, Liu X-X, Diamond D, Lau KT (2011) Synthesis of electrochemically-reduced graphene oxide film with controllable size and thickness and its use in supercapacitor. Carbon 49:3488–3496. https://doi.org/10.1016/j.carbon.2011.04.047
Li W, Liu J, Yan C (2013) Reduced graphene oxide with tunable C/O ratio and its activity towards vanadium redox pairs for an all vanadium redox flow battery. Carbon 55:313–320. https://doi.org/10.1016/j.carbon.2012.12.069
Some S, Kim Y, Yoon Y, Yoo H, Lee S, Park Y, Lee H (2013) High-quality reduced graphene oxide by a dual-function chemical reduction and healing process. Sci Rep 3:1–5. https://doi.org/10.1038/srep01929
Zhao F, Dong B, Gao R, Su G, Liu W, Shi L, Xia C, Cao L (2015) A three-dimensional graphene-TiO2 nanotube nanocomposite with exceptional photocatalytic activity for dye degradation. App Surf Sci 351:303–308. https://doi.org/10.1016/j.apsusc.2015.05.121
Chen W, Yan L, Bangal PR (2010) Chemical reduction of graphene oxide to graphene by sulfur-containing compounds. J Phys Chem C 114:19885–19890. https://doi.org/10.1021/jp107131v
Zhang J, Yang H, Shen G, Cheng P, Zhang J, Guo S (2010) Reduction of graphene oxide via L-ascorbic acid. Chem Commun 46:1112–1114. https://doi.org/10.1039/b917705a
Aawani E, Memarian N, Dizaji HR (2018) Synthesis and characterization of reduced graphene oxide–V2O5 nanocomposite for enhanced photocatalytic activity under different types of irradiation. J Phys Chem Solids 125:8–15. https://doi.org/10.1016/j.jpcs.2018.09.028
Iskandar F, Hikmah U, Stavila E, Aimon AH (2017) Microwave-assisted reduction method under nitrogen atmosphere for synthesis and electrical conductivity improvement of reduced graphene oxide (rGO). RSC Adv 7:52391–52397. https://doi.org/10.1039/c7ra10013b
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565. https://doi.org/10.1016/j.carbon.2007.02.034
Park S, An J, Potts JR, Velamakanni A, Murali S, Ruoff RS (2011) Hydrazine-reduction of graphite-and graphene oxide. Carbon 49:3019–3023. https://doi.org/10.1016/j.carbon.2011.02.071
Fernández-Merino MJ, Guardia L, Paredes JI, Villar-Rodil S, Solís-Fernández P, Martínez-Alonso A, Tascón JMD (2010) Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J Phys Chem C 114:6426–6432. https://doi.org/10.1021/jp100603h
Abdolhosseinzadeh S, Asgharzadeh H, Seop Kim H (2015) Fast and fully-scalable synthesis of reduced graphene oxide. Sci Rep 5:1–7. https://doi.org/10.1038/srep10160
Cobos M, González B, Fernández MJ, Fernández MD (2018) Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int J Biol Macromol 114:599–613. https://doi.org/10.1016/j.ijbiomac.2018.03.129
Lin L, Peng H, Liu Z (2019) Synthesis challenges for graphene industry. Nat Mat 18:520–524. https://doi.org/10.1038/s41563-019-0341-4
Zhu Y, Ji H, Cheng H-M, Ruoff RS (2018) Mass production and industrial applications of graphene materials. Natl Sci Rev 5:90–101. https://doi.org/10.1093/nsr/nwx055
“Top Graphene Companies and Manufacturers in the USA and Globally”. Retrieved from https://www.thomasnet.com/articles/top-suppliers/graphene-companies-manufacturers/#graphenemanufacturers on January 3rd 2021
Acknowledgements
J.C.D. thanks O.F.O.A. for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Dale Huber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gutiérrez-Cruz, A., Ruiz-Hernández, A.R., Vega-Clemente, J.F. et al. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J Mater Sci 57, 14543–14578 (2022). https://doi.org/10.1007/s10853-022-07514-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07514-z