Abstract
The effect of CuO addition on the properties of (Co, Nb, Cr)-doped SnO2 varistors were investigated. The samples with different CuO concentrations were fabricated by the conventional ceramic method and sintered at 1,200, 1,250, 1,300 and 1,350 °C for 2 h. It is found that the nonlinear coefficient presents a peak value at 0.2 mol % CuO addition. The leakage current density decreases with increasing CuO from 0 to 0.2 mol %, and then increases when the concentration of CuO is above 0.2 mol %. The breakdown electrical field decreases from 356 to 248 V/mm with increasing CuO from 0 to 0.6 mol %. The optimal samples obtained by doping CuO with 0.2 mol % and sintered at 1,300 °C have the highest nonlinear coefficient value of 31 and the lowest leakage current density of 2 μA/cm2.
Similar content being viewed by others
1 Introduction
The rutile crystalline structural SnO2 is an n-type semiconductor with many interesting electronic properties. In the past, it is widely used in gas sensors and wet sensors. However, since it is first studied as varistors by the group of Pianaro, a great number of papers on the new SnO2-based varistors have been published [1–4]. Compared with the commercial ZnO varistors, it has good thermal conductivity and high temperature resistance. The main characteristic of this new varistor system is its simple microstructure, which has only one phase with X-ray precision. The single-phase microstructure of SnO2 based varistors maybe resolve the problem of the degradation of the commercial ZnO–Bi2O3 based varistors.
In the past decades, many dopants have been studied, such as ZnO, CoO, MnO, Nb2O5, CuO, Ta2O5, Cr2O3, Al2O3 [5–13]. And the effects of these dopants are different in SnO2-based varistor. ZnO, CoO, MnO have significant role to improve the density, Nb2O5 and Ta2O5 mainly improve the conductivity, and Cr2O3 and Al2O3 mainly improve current–voltage characteristic of SnO2-based ceramics. From the recent publications, CoO, Nb2O3 and Cr2O3 doped SnO2-based varistors show the excellent nonlinearity and high density [4, 14–17].
In the previous works [18, 19], the effects of CuO on the grain size, density, nonlinearity, and dielectric property of the SnO2–Ni2O3–Nb2O5 and SnO2–Co2O3–Ta2O5 varistors were investigated. In this work, the effects of CuO addition and sintering temperature on the microstructure and electrical properties of the Co2O3–Nb2O3–Cr2O3 doped SnO2 varistors were investigated in detail. It is found that CuO addition and sintering temperature could evidently adjust the microstructure, and as a result, the breakdown electrical field decreases, the nonlinearity improves and the leakage current decreases.
2 Experimental
Five raw materials of analytic level, SnO2 (99.8 %), Co2O3 (99 %), Nb2O5 (99.5 %), CuO (99 %) and Cr2O3 (99 %), were used to prepare the SnO2 based varistors. The varistors were fabricated by a conventional electrical ceramics processing method. The compositions in molar ratios were (98.2 − X) % SnO2 + 0.75 % Co2O3 + 0.05 % Nb2O5 + 0.05 % Cr2O3 + X % CuO, with X = 0.0, 0.1, 0.2, 0.4 and 0.6 mol %. The mixtures of raw materials were milled in nylon kettle for 4 h at 800 r/min with ZrO2 balls and distilled water, the ratio of balls to the mixed powders and water was 2:1:1. Then as milled slurry was dried, mixed with 0.8 wt % of PVA binder and pressed into pellets (10.00 mm × 1.32 mm) by uniaxial pressing (2.5 MPa). After burning out the PVA binder at 650 °C for 2 h, the pellets were respectively sintered at 1,200, 1,250, 1,300, and 1,350 °C for 2 h, and then cooled to the room temperature at a rate of 5 °C/min.
The microstructures of the surfaces were analyzed by scanning electron microscopy (SEM, Model JSM-5610LV, JEOL), and the phase composition was determined by X-ray powder diffraction (XRD). The grain sizes (d) were calculated by microstructure linear analysis [20]. The sintered density (ρ) of ceramics was measured by the Archimedes method in distilled water. For the electrical measurements, silver electrodes (0.5 cm2) were made on both surfaces of the sintered pellets, after which the pellets were heat-treated at 600 °C for 2 h. The I–V characteristics were measured by using a DC parameter instrument for varistors (Model CJ1001). The voltage gradient (V 1mA) was determined at a current of 1 mA and nonlinear coefficient (α) was obtained from the equation of \(\alpha = {\text{l}}/{ \lg }\left( {V_{\text{lmA}} /V_{{ 0. {\text{lmA}}}} } \right)\). The leakage current (I L ) was measured at 0.75V1mA.
3 Results and discussion
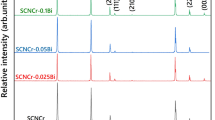
Figure 1 illustrates the XRD patterns of the as-prepared SnO2-based varistor ceramics with different amounts of CuO doping sintered at 1,300 °C for 2 h. There is no any second phase detected in all samples except the SnO2 rutile phase, which is consistent with the former work [10]. The same trend is observed for samples sintered at 1,200, 1,250, and 1,350 °C. All dopants introduced to the SnO2 matrix form a stable solid solution according to Eqs. (1), (2), (3) and (4).
The XRD patterns between the 2θ = 26–27° of the sintered products contained different amount of CuO are shown in Fig. 2. It is important to note that the peaks of the SnO2 diffraction peaks shift towards lower angles with increasing of CuO content, indicating that the lattice parameter of the as-sintered SnO2 increases with increasing of CuO content. This is attributable to the solid solution formation between SnO2 and CuO. The ionic crystal radii of Sn4+ and Cu2+ are 0.69 and 0.73 Å, respectively. Consequently, one can conclude that the Sn site of SnO2 lattice could be easily substituted by Cu to form substitutional limited solid solutions. The lattice parameters of the as-sintered Sn1−x Cu x O samples as a function of CuO content are shown in Fig. 3. It could be observed that both a- and c-axis of the SnO2 increase as the amount of CuO increases from 0 to 0.6 mol %. Especially, the a-axis decreases more evidently from 4.7179 to 4.7778 Å by 1.26 %, which is obeyed the generally known Vegard’s law.
The effect of CuO concentration on the microstructure of the samples is shown in Fig. 4. It reveals that the surfaces of all as fabricated products possess a plain texture with the expected clear grain boundary for ceramic materials. The grain clearance of the samples with 0.2 mol % CuO added is much smaller than those in the samples without CuO and with 0.6 mol % CuO addition. The grain distribution of the samples with 0.2 mol % CuO is also the most homogeneous. The SnO2 grain size gently increases from 4.6 to 5.0 with the increasing amount of the CuO content from 0 to 0.2 mol %. But it increases evidently from 5.0 to 6.2 with increasing amount of CuO content from 0.2 to 0.6 mol %. It is indicated that few amount of CuO addition mainly improves the density (shown in Table 1) and uniform dispersion of the microstructure of the resultant samples. However, an excess of CuO addition promotes grain growth obviously. That is because CuO will melt at high temperature. It is the liquid CuO at the grain boundary that proved the uniform distribution of dopant and density of the SnO2 samples. The SnO2 grain size increases with the overmuch addition of CuO because the substitution of Cu2+ for Sn4+ increases the activity of SnO2 by means of distortion of the SnO2 lattice, as discussed in the XRD test. In addition, as seen in the Fig. 5, when the sintering temperature increases to 1,300 °C, the structure is very dense and the grain boundary is very clear, which implies that the SnO2 grains are well crystallized with good integrality. The grain size grows from 2.8 to 5.0 with an average value. The sintered density of the samples increased from 6.55 to 6.62 g/cm3 corresponding to 94.2–95.3 % of the theoretical density (TD) (pure SnO2, TD = 6.95 g/cm3) when the sintering temperature increases from 1,200 to 1,300 °C. However, the sintered density has little decrease when the sintering temperature up to 1,350 °C, it attributed to the volatility of the liquid at high temperatures. The details are shown in Table 2.
The electrical characteristics of the as fabricated SnO2-based varistors as a function of CuO concentration sintered at 1,300 °C are plotted in Fig. 6. All the samples have excellent I–V curves in the figures. The breakdown electrical filed decreases from 356 to 248 V/mm with increasing CuO concentration from 0 to 0.6 mol %, which can be explained according to the Eq. (5):
where n is the average grain number per unit length, V gb, about 2.2–3 V [21], is the breakdown voltage of a grain boundary. As shown in Fig. 4, the average grain size of the sintered samples increases with the increase of the CuO concentration, as a result, the breakdown electrical filed, E B, decreases. And the breakdown electrical filed decreases from 664 to 253 V/mm with increasing sintering temperature from 1,200 to 1,350 °C, as shown in Fig. 7.
Figure 8 shows that the nonlinear coefficient and leakage current density of the samples with different CuO concentration sintered at 1,300 °C. The nonlinear coefficient has a peak value of 31 when added 0.2 mol % CuO. As the CuO concentration increases above 0.2 mol %, the nonlinear coefficient decreases. According to the previous work [22], the decreasing nonlinear coefficient might be associated to the reduction of the effective grain boundary. On the contrary, The leakage current density decreases firstly with the CuO concentration increasing from 0 to 0.2 mol %, and then increases gradually with the CuO concentration further increasing from 0.2 to 0.6 mol %. As shown in Fig. 4, the high density and uniform microstructure result in lowing the leakage current density of the 0.2 mol % CuO doped SnO2 samples.
Figure 9 shows the nonlinear coefficient and leakage current density of the 0.2 mol % CuO added samples sintered at different temperatures. The nonlinear of the obtained samples is better than that at any other sintering temperature. Meanwhile, the sample sintered at 1,300 °C presents a lowest leakage current density value of 2 μA/cm2. As shown in Fig. 5, when the samples with 0.2 mol % CuO addition are sintered at, the product possesses the highest dense and most homogeneous microstructure, which lead to a low leakage current density. Based above results, it can be sure that the 0.2 mol % CuO is the optimum concentration, and 1,300 °C is a appropriate temperature for synthesis of the SnO2 based varistors.
4 Conclusions
The effect of CuO addition on the microstructure and electrical properties of SnO2 varistors were studied in detail at different sintering temperatures. The breakdown electrical filed of the SnO2 varistors decreases with increasing CuO content. While the nonlinear coefficient possesses a peak value of 31 and the leakage current density has the minimum value of 2 μA/cm2 at 0.2 mol % CuO sintered at 1,300 °C. Hence, the 0.2 mol % is the optimum CuO content for SnO2 varistors, and 1,300 °C is the best sintering temperature for this system.
References
S.A. Pianaro, P.R. Bueno, E. Longo, J.A. Varela, J. Mater. Sci. Lett. 14, 692 (1995)
J.A. Cerri, E.R. Leite, D. Gouvea, E. Longo, J.A. Varela, J. Am. Ceram. Soc. 79, 799 (1996)
R. Metz, D. Koumeir, J. Morel, J. Pansiot, J. Eur. Ceram. Soc. 28, 829 (2008)
M.A. Ramirez, J.F. Fernandez, M. De la Rubia, J. De Frutos, P.R. Bueno, E. Longo, J.A. Varela, J. Mater. Sci. Mater. Electron 20, 49 (2009)
L. Perazolli, A.Z. Simoes, U. Coleto Jr, J.A. Varela, Meter. Lett. 59, 1859 (2005)
G. Brankovic, Z. Brankovic, M.R. Davolos, M. Cilense, J.A. Varela, Mater. Charact. 52, 243 (2004)
W.K. Bacelar, P.R. Bueno, E.R. Leite, E. Longo, J.A. Varela, J. Eur. Ceram. Soc. 26, 1221 (2006)
R. Metz, D. Koumeir, J. Morel, J. Pansiot, J. Eur. Ceram. Soc. 28, 829 (2008)
M.O. Orlandi, M.R.D. Bomio, E. Longo, P.R. Bueno, J. Appl. Phys. 96, 3811 (2004)
A.V. Gaponov, A.B. Glot, J. Mater. Sci. Mater. Electron 21, 331 (2010)
F.M. Filho, A.Z. Simoes, A. Ries, E.C. Souza, L. Perazolli, M. Cilense, E. Longo, J.A. Varela, Ceram. Int. 31, 399 (2005)
W.K. Bacelar, P.R. Bueno, E.R. Leite, E. Longo, J.A. Varela, J. Eur. Ceram. Soc. 26, 1221 (2006)
A. Mosquera, J.E.R. Paez, J.A. Varela, P.R. Bueno, J. Eur. Ceram. Soc. 27, 3893 (2007)
M.R.C. Stantos, V.C. Sousa, M.M. Oliveira, F.R. Sensato, W.K. Bacelar, J.W. Gomes, Mat. Chem. Phys. 90, 1 (2005)
G. Brankovic, Z. Brankovic, J.A. Varela, J. Eur. Ceram. Soc. 25, 3011 (2005)
P.A. Santos, S. Maruchin, G.F. Menegoto, A.J. Zara, S.A. Pianaro, Meter. Lett. 60, 1554 (2006)
M.L. Moreira, S.A. Pianaro, A.V.C. Andrade, A.J. Zara, Mater. Charact. 57, 193 (2006)
W.X. Wang, J.F. Wang, H.C. Chen, W.B. Su, G.Z. Zang, Mater. Sci. Eng. B 99, 457 (2003)
C.M. Wang, J.F. Wang, H.C. Chen, W.B. Su, G.Z. Zang, P. Qi, M.L. Zhao, Mater. Sci. Eng. B 116, 54 (2005)
M.I. Mendelson, J. Am. Ceram. Soc. 52, 443 (1969)
E.R. Leite, A.M. Nascimento, P.R. Bueno, J. Mater. Sci. Mater. Electron 10, 321 (1999)
J.S. Vasconcelos, N.S. Vasconcelos, M.O. Orlandi, P.R. Bueno, Appl. Phys. Lett. 89, 152102 (2006)
Acknowledgments
This work was supported by the Scientific and technological project of Wenzhou (H20100079, H20100087), Special project of Shaanxi provincial education department (11JK0810) and the Graduate Innovation Fund of Shaanxi University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hu, G., Zhu, J., Yang, H. et al. Effect of CuO addition on the microstructure and electrical properties of SnO2-based varistor. J Mater Sci: Mater Electron 24, 2944–2949 (2013). https://doi.org/10.1007/s10854-013-1195-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1195-1