Abstract
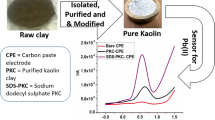
A new electrode based on carbon paste (CP) modified with natural clay was used for heavy metals detection. The investigation of the raw clay material (X-ray diffraction, infrared spectroscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy, thermal analysis and particle size distribution) revealed different morpho-structural properties of the natural material. The electrochemical behaviour of the modified carbon paste electrode and its ability to detect heavy metals (Zn2+, Cd2+, and Cu2+) was studied by different electrochemical techniques like cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and square wave anodic stripping voltammetry (SWASV). Both electrochemical and analytical parameters of the investigated modified electrode recommend them as stable, sensitive and reproducible sensors for individual heavy metals detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last period, heavy metals detection and their analytical determination were the subject of various studies, because of their significant influences on human health, animal health, and the environment [1, 2]. Also, many worldwide or European organizations and committees (e.g., European Parliament [3, 4], US Environmental Protection Agency (EPA) [5] or the World Health Organization (WHO) [6]) give great importance to heavy metal ions detection (e.g., Pb2+, Ni2+, Cd2+ and Cu2+), which were included in the list of emerging elements in the domain of water quality. Pointing to achieve fast, precise, and reliable heavy metals determination, various methods, like inductively coupled plasma-mass spectroscopy (ICP-MS), inductively coupled plasma/atomic emission spectrometry (ICP-AES), inductively coupled plasma-optical emission spectrometry (ICP-OES), ion chromatography ultraviolet-visible spectroscopy (IC-UV-vis), atomic absorption spectroscopy (AAS), capillary electrophoresis (CE), colorimetric detection, spectrophotometry, fluorometry, volumetric analysis, chemiluminometry, chromatography [7], and electrochemical detection [8, 9] were reported in the literature.
A comparison of the aforementioned approaches reveals that the electrochemical methods [10] are less expensive and offer a fast response and necessary characteristics for growing efficiency. Furthermore, there are significant advantages, such as simplicity, intrinsic sensitivity and great selectivity, which indicate that electrochemical methods are among the most promising options for heavy metals detection.
The first electrochemical sensors applied for heavy metal detection were mercury-based [11], which has several advantages such as ensuring a clean surface which is easy to reproduce and leads to competitive electrochemical properties. Besides the high toxicity of Hg, other inconveniences like volatility, difficult procedure, the difficulty for miniaturization, and achievement price, determine the employment of various other materials types, as following metals or metal nanoparticles (NPs) (Au, Ag, Ir, Sb, St, and Bi) [8, 9, 12], metal oxide nanoparticles [13,14,15], carbonaceous nanomaterials [8, 9, 16], silicon-based materials [17], and clays [18].
In the last 65 years, since Adams published the first carbon paste electrode [19], a wide range of carbon paste electrodes with various modifiers have been developed for many applications. In previously published articles, the modified electrodes are obtained following a complicated time and energy-consuming procedure or employing expensive materials.
In this context, the use of raw, natural materials (for example clays) having well electrochemical features, such as potential range [20], high stability, low costs and simplicity of handling, as well as low toxicity and ecological aspects are highly recommended. As a result of these factors, claybased modified electrodes emerged as viable alternatives for constructing electrochemical sensors as an alternative to Hg electrodes or other sophisticated and costly electrodes. It is important to mention that because of adsorption power and good ion exchange capacity [21], clays have been used either as a dopant [22] or as organic–inorganic hybrid materials [22,23,24] in order to modify the carbon paste electrodes. Clay-modified carbon paste electrodes consist of solid graphite particles, an organic pasting agent (i.e., oil), solid clay particles, and a water phase incorporated in the clay structure, generating a very complex, heterogeneous system that is difficult to characterise. The most recent examples of using clay-based carbon paste electrodes (CPE) are those using United Arab Emirates clays sintered at 1000 °C [25, 26] or Fe/ZSM-5 zeolite synthesised by the hydrothermal method at 170 °C [26], which were used for Cr6+ [26], or Pb2+, Cu2+, Cd2+, and Hg2+ [25] detection, respectively. Also, zeolite membrane was prepared by modifying natural Moroccan diatomite to produce a zeolite type LTA by a hydrothermal process at 80 °C [27]. The key challenges of these tools are (i) the diversity in composition as a function of origin, sampling and pretreatment applied, and (ii) the obtaining of a homogeneous paste. Contrarily with the examples mentioned before, the use of raw, natural materials, without any treatment, could be advantageous in terms of the reproducibility of the final device [28]. However, powerful and useful tools as modified carbon paste electrodes remain popular due to their low cost and enhanced sensibility.
Thus, the novelty lies in the use of untreated natural clay, unlike most previous studies involving modified or treated clays to enhance their properties for better adsorption and sensitivity. The use of natural clay directly involves exploring the inherent properties of clay, such as its natural adsorption capacities and ion exchange capabilities, which could uniquely interact with the carbon paste matrix to detect low concentrations of heavy metals. To the best of our knowledge, the present work reports, for the first time, the use of a new raw natural clay from Cote d’Ivoire, region Adiaho (ADI), without any modification (functionalization, activation, or other chemical or thermal treatments) for the preparation of modified carbon paste electrodes (CPE-x%ADI, where x represents the percentage of clay in the solid part of the modified carbon paste). After the morpho-structural characterization of the raw natural clay was performed, its abilities to improve sensitivity due to an enhanced charge transfer process were exploited. Thus, the developed CPE-x%ADI modified electrode was used for individual heavy metal detection in synthetic and real water samples. The obtained electroanalytical parameters of the clay-based electrode show that the eco-friendly investigated new clay can be used in an alternative miniaturised, portable, and mass-production of very inexpensive, simple, fast, and reproducible screen-printed electrodes, compared with other expensive and complex electrode materials previously listed.
Experimental
Reagents
Graphite powder (Fluka) and Paraffin oil (Aldrich) were used for preparing carbon paste electrodes (CPE). Appropriate quantities of acetic acid and sodium acetate (Merck, Darmstadt, Germany) were dissolved in distilled water for the buffer solution preparation (0.1 M, pH 4.5). In order to adjust the pH of the buffer solution, acetic acid or KOH solutions (Merck, Darmstadt, Germany) were added. A 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] (from Reactivul, Bucuresti, Romania) in 0.1 M KCl (Merck, Germany) solution was used as a redox probe.
Heavy metals solutions were prepared by dissolving appropriate amounts of Cd(NO3)2 × 4H2O, Zn(NO3)2 × 6H2O and CuSO4 × 5H2O (Reactivul, Bucuresti, Romania) in acetate buffer. All chemicals were of analytical degree and were used as received.
Morpho-structural measurements
X-ray diffraction (XRD) patterns were recorded at a Bruker D8 Advance diffractometer, with a Cu tube (Kα1 with a wavelength of 0.15418 nm), Ni filter and a LynxEye detector.
The FTIR spectrum of samples was recorded on a Nicolet 6700 Spectrometer (KBr pellets technique).
Scanning electron microscopy (SEM) was performed using a SU8230 scanning electron microscope (Hitachi, Japan). Spectra of energy-dispersive X-ray spectroscopy (EDXS) and chemical maps for the elements were acquired using a Dual EDX System (X-Max N100TLE Silicon Drift Detector) from Oxford Instruments.
The thermal analysis (TG and DTA) was performed using a SDT Q600 type instrument, in air up to 1000 °C at 10 °C min−1 rate and alumina standards.
A Shimadzu Sald-7101 (Japan) micro- and nanoparticle size analyser was used for investigating the particle size distribution (PSD) of clay material powders. The sample was immersed for 15 s in distilled water used as a solvent, with aggregation being reduced using treatment with ultrasounds.
The chemical composition determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) and flame atomic emission spectroscopy (FAES) method was performed using a SPECTRO CIROSCCD spectrometer (Spectro, Kleve, Germany). Na and K were determined using the Sherwood model 360 flame photometer (Cambridge, United Kingdom). For As determination, the SPECTRO CIROSCCD spectrometer was interfaced with the online HGX-200 hydride generator (Teledyne CETAC, Nebraska, USA). Standardised protocols were used for sample preparation [29, 30] and operating conditions [31]. Linear calibrations for each ion were performed and the detection limits obtained were calculated using the 3σ criterion from repeated measurements of the blank solution and considering the sample preparation protocol [32]. The results are expressed as concentration ± C.I. a (mg × kg−1), where C.I. is the confidence interval for n = 3 measurements for each sample and 95% confidence level [32].
Electrochemical measurements
All electrochemical measurements (cyclic voltammetry, electrochemical impedance spectroscopy and square wave anodic stripping voltammetry) were performed using a PC-controlled electrochemical analyzer (AUTOLAB PGSTAT302N EcoChemie and AUTOLAB PGSTAT 10, Utrecht, Netherlands). The clay-modified carbon paste electrode (CPE-x%ADI, geometric area ~ 0.07065 cm2), as the working electrode, Pt wire, as the auxiliary electrode, and Ag|AgCl, KClsat as the reference electrode equipped the conventional three-electrodes set-up. The SWASV investigations were realised in 0.1 M acetate buffer (pH 4.5) in the presence or absence of heavy metal ions using the following operational parameters: (i) preconcentration at a potential of − 1.2 V versus Ag/AgCl, KClsat), under continuous stirring at 400 rpm, during 180 s, (ii) equilibration for 10 s of the unstirred solution and (iii) anodic stripping between − 1.2 V and + 0.2 V Ag/AgCl, KClsat, at frequency of 10 Hz, amplitude of 20 mV, step potential of 3 mV.
All electrochemical investigations were realised in aerated solution at ambient conditions.
Preparation of CPE-x%ADI
The clay-modified carbon paste electrode (CPE) was prepared by thoroughly mixing the required amounts of graphite powder with clay and paraffin oil, respecting the solid (graphite and clay): liquid (oil) percentage mass ratio of 70%: 30%. The components were combined using a mortar and pestle until a homogeneous paste was obtained. Thus, different mixtures (containing 5, 10, 15, 20, 25% clay in the solid part, or weight ratio clay: graphite of 1:13, 2:12, 3:11, 4:10, 5:9) were prepared (See Supplementary Information Table S1). The obtained electrodes were symbolised CPE-x%ADI, where x represents the percentage of clay in the solid part of the modified carbon paste.
The achieved paste was incorporated into the cavity of a Teflon holder. In order to ensure the electric contact, the other side of the Teflon holder has a piece of copper. A new electrode surface can be obtained by removing ~ 2 mm of the superficial layer of paste and replacing it with a new quantity of fresh paste.
Real sample analysis
The Water Company SA (Alba, Romania) delivers three samples from drilling water (Rosia Poieni, Alba, Romania) analysed by the standardized method (i.e., atomic absorption spectroscopy, AAS) using the AVANTA PM (by GBC Scientific Equipment) spectrometer. Each sample had one heavy metal ion in high concentration, and the other ions were below the detection limit of the atomic absorption spectrometer. The results, obtained using the clay-based modified electrode by standard addition method, were compared with those obtained by AAS.
Results and discussions
Morpho-structural characterization
The recorded diffractogram of the raw ADI clay (Fig. 1A) displays the presence of characteristic peaks attributed to quartz (Q), as major phase, with kaolinite (K) and muscovite (Mu) as minor phases, according to Joint Committee on Powder Diffraction Standards (JCPDS) (see inset Fig. 1A). Smectite (S)—most likely montmorillonite—as clay phase, but component like plagioclase feldspar (P) as associated mineral [22] could also be present in the analysed sample. Their identification is challenging due to different overlaps [22]. These results are consistent with previously reported data for other types of clays [18], particularly those originating from Cote d’Ivoire [33,34,35].
The FTIR spectrum of raw ADI clay presents four vibrational regions (Fig. 1B), which contain characteristic features of the clay’s spectrum. Thus, the four bands characteristic of O–H stretching vibration (3696, 3650, 3619 and 3442 cm−1), Si–O stretching (1104–1007 cm−1), Al–O bending (912 cm−1), and the SiO2 bending (537–467 cm−1) regions are well evidenced. These bands are usually present in kaolinite, a common clay mineral, also. Moreover, O–P=O and O–P–O bending vibration at 796 and 695 cm−1, respectively, are present [35, 36].
While the FTIR spectrum alone does not provide precise information regarding the exact composition or the proportion of different minerals present in the sample, the FTIR results corroborated with XRD results tend to confirm that clay minerals (quartz, kaolinite, muscovite, montmorillonite) represent the components of ADI clay.
SEM image of raw ADI clay (Fig. 1C) shows the tendency of the clay’s components to form aggregates of well-crystallized particles forming layered sheets of the clay [18, 37].
The TG and DTA curves of raw ADI clay are presented in Fig. 1E. The TG curve indicates three weight loss steps caused by endothermic processes: first moisture release of 4.07% at 62 °C, a second decomposition step with a mass loss of 2.53% (small endothermic peak at 219 °C), and a structural water release (i.e., 6.15% at 360–630 °C). At 300 °C, an exothermic process of transformation of goethite into hematite took place [38]. At 573 °C a dehydroxylation transformation occurs followed by the elimination of gas residues resulting from previous processes (at 630–1190 °C). The dehydroxylation due to the loss of -OH lattice of water occurs at different temperatures in the function of particle size and variations in crystallinity [22, 39]. At 947 °C, an exothermic process attributed to the formation of a new compound can be observed, followed by the endothermic melting of different components.
The particle size distribution (PSD) of clay minerals represents an important parameter for mineral identification [21]. The PSD of the clay sample in suspension analyzed by laser diffraction is given in Fig. 1F. As shown, a wide PSD of the initial powder consists of a one-grain population with a mean value of 4.222 μm (standard deviation, SD = 0.440). The D25, D50 and D75 values were 2.016, 4.126 and 8.663 μm for the full range 0.503–31.057 μm clay powder. The wide particle size distribution ranging from 0.503 to 31.057 μm, mean particle size, and percentile values of the clay sample are important for optimizing the use of clay as a modifying agent in carbon paste electrodes. The availability of particles with different sizes can be advantageous for tailoring the electrode’s properties to specific analytical needs. In addition, the narrowness of the standard deviation (SD = 0.440) indicates that the particle size distribution is relatively uniform, which can contribute to the reproducibility of results in electroanalytical experiments.
The chemical composition determined by ICP-OES and FAES method shows that the ADI clay contains the following major elements: Al (123200 ± 18200 mg kg−1), Ca (1930 ± 260 mg kg−1), Fe (53400 ± 3200 mg kg−1), Mg (4080 ± 170 mg kg−1), Si (89500 ± 6400 mg kg−1), Na (1900 ± 190 mg kg−1), K (6550 ± 1010 mg kg−1), and the following mi-nor elements as Ba (442 ± 39 mg kg−1), Cd (27.6 ± 3.8 mg kg−1), Co (202 ± 51 mg kg−1), Cr (133 ± 9 mg kg−1), Cu (33.5 ± 5.5 mg kg−1), Mn (376 ± 9 mg kg−1), Ni (70 ± 7 mg kg−1), Pb (430 ± 35 mg kg−1), Ti (10550 ± 910 mg kg−1), Zn (46.5 ± 5.4 mg kg−1), P (551 ± 52 mg kg−1), S (241 ± 7 mg kg−1), As (5.61 ± 0.75 mg kg−1). The significant presence of elements like Al and Fe in the clay suggests that it may have substantial adsorption capacity for heavy metal ions, including Cd, Zn, and Cu, making ADI clay a potentially effective modifying agent for enhancing the adsorption and preconcentration of these metals.
Electrochemical behaviour of the CPE-x%ADI modified electrode
The electrochemical activity of the prepared CPE-x%ADI modified electrodes was studied by cyclic voltammetry, in a 5 mM solution of K3[Fe(CN)6]/K4[Fe(CN)6] in 0.1 M KCl, at 50 mV/s. The presence of a peak pair corresponding to the redox probe behaviour, having the potentials placed between + 0.035 and + 0.375 V versus Ag/AgCl, KClsat was evidenced (Fig. 2).
The electrochemical parameters estimated for the different investigated CPE-x%ADI modified electrodes as follows: anodic and cathodic peak potential (Epa, Epc), anodic and cathodic peak intensity (Ipa, Ipc), difference between anodic and cathodic peak potential (ΔEp = Epa–Epc), formal standard potential (E0’ = (Epa + Epc)/2)), and the Ipa /Ipc ratio are summarised in Table 1. As seen, the obtained results confirm that the studied redox process has a quasi-reversible behavior (ΔEp > 60 mV, Ipa/Ipc = 1) [40].
The values of the electrochemical parameters showed that ΔEp for clay-modified electrodes (CPE-x%ADI) increases compared to the bare electrode (CPE), as the percentage of clay (x%) in the solid mass increases. The greater this difference, the more quasi-reversible the studied redox process becomes. This behavior can be explained by the fact that the amount of clay, which is less conductive, increases in the electrode matrix. Also, it must be stated that the higher peak intensity of the current is recorded for the CPE-15%ADI-modified electrode matrix. Therefore, the CPE-15%ADI-modified electrodes with proven optimal composition were used in further experiments.
Scan rate influence
Valuable information about the mechanism of the electrochemical process of a redox probe can be obtained by investigating the influence of the scan rate on the peak current intensity.
Thus, the recorded CVs at CPE-15%ADI (Fig. 3A) proved that with the increase of the scan rate, there is an increase in the intensity of the anodic and cathodic peak currents, and a shift of the peak potentials towards more positive and negative values, respectively.
In order to prove that the redox process presented in Fig. 3A occurs under con-trolled diffusion, the validity of the Randles–Sevcik Eq. (1) must be verified [40]:
where: Ip is the peak current (in A); n is the number of electrons transferred in the redox process (in our case 1); A represents the electrode area (in cm2); D is the diffusion coefficient (in cm2/s, in our case D = 6.5 × 10−6 cm2/s [41]); C is the concentration of the redox species (in mol/cm3, in our case C = 5 × 10−6 mol/cm3); ν is the scan rate (in V/s).
Consequently, the log Ip–log v for each electrode type was plotted (e.g., Fig. 3B), obtaining the following linear regressions equations in the case of CPE-15%ADI modified electrode:
Since the slope of the log Ip–log v dependence (Table 2) has a value very close to the theoretical value of 0.5, it can be established that, as expected for the studied redox process, it occurs under diffusive control.
Also, based on Eq. (1), from the slope of representations I versus v1/2 the electrochemically active surface of the modified electrodes can be estimated.
The obtained results (Table 2) confirm that the presence of 15% clay in the electrode composition (i.e., CPE-15%ADI modified electrode) increases the active surface area 5 times compared to the unmodified CPE electrode and approximately 2 times compared to the other modified electrodes containing different percents of clay. Consequently, the CPE-15%ADI-modified electrode will be used for further quantitative measurements.
EIS measurements
Electrochemical impedance spectroscopy (EIS) was performed in the presence of the [Fe(CN)6]4−/3− redox couple in order to evaluate the activity of the CPE-15%ADI modified electrode and the effect of clay presence in the modifier electrode material. The Nyquist plots presented in Fig. 4 were recorded at CPE and CPE-15%ADI modified electrodes in a 0.1 M KCl solution containing 5 mM K3[Fe(CN)6]/ K4[Fe(CN)6] in a frequency range of 10−1–104 Hz.
Figure 4 shows that both electrodes exhibit (i) at high frequencies well-defined semicircles characteristic for a faradaic process controlled by charge transfer phenomena, and (ii) at low frequency a straight line where the electrochemical process is controlled by mass transfer phenomena. The obtained experimental data were fitted with Rs(Qdl(RctW)) and Rs(Qdl(Rct(Qclay(RclayW))) equivalent electric circuits for CPE and CPE-15%ADI, respectively (see inset Fig. 4). Both circuits contain resistors (Rs, Rclay, Rct), constant phase elements (Qclay, Qdl) and Warburg impedance to explain mass transfer resistance. The charge transfer resistance (Rct) exhibits a significant reduction from 1414 ± 1.5 ohms in the unmodified CPE to 792.1 ± 1.14 ohms in the CPE-15%ADI. This reduction underscores an enhanced electron transfer capability in the modified electrode, likely due to either an increase in the effective surface area or the catalytic effects of metal oxides within the clay matrix [42].
Optimization of experimental parameters
In order to obtain the best values for the peak current, the optimization of the deposition time, potential of deposition, frequency and amplitude of the SWASV method were studied in a 5 × 10−6 M Cd2+ solution (Fig. 5A–D). The deposition time for the accumulation of Cd2+ on the electrode surface was studied in the interval of 60–300 s, at an applied potential of − 1.4 V versus Ag/AgCl, KClsat.
Influence of deposition time (A), deposition potential (B), frequency (C) and amplitude (D) on the detection of 5 × 10−6 M Cd2+ at CPE-15%ADI modified electrode. Experimental conditions: electrolyte, 0,1 M acetate buffer (pH 4.5); frequency, 10 Hz (A, B, D); amplitude, 20 mV (A, B, C); step potential 3 mV; deposition time, 180 s (B, C, D); deposition potential, − 1.2 V versus Ag/AgCl, KClsat (A, C, D), starting potential, − 1.2 V versus Ag/AgCl, KClsat.
The intensity of the peak current has a linear dependency on the deposition time (Fig. 5A). Because in routine measurements, the best choice is a compromise between sensitive analysis and reasonable analysis time, 180 s for deposition time was chosen as optimum, in comparison with other works [8, 9, 16]. The evolution of the peak current against the deposition potential, at 180 s, was studied in the interval of − 1.5 to − 1 V versus Ag/AgCl, KClsat, showing a maximum at − 1.2 V versus Ag/AgCl, KClsat (Fig. 5B), the value which was further used in order to enhance the mass transfer rate and which allows the metallic cations to reach the electrode surface in the preconcentrating step of analysis [43].
The influence of operational parameters of SWASV, i.e., frequency and amplitude were studied in the range of 8–100 Hz and 10–200 mV, respectively, at a deposition potential of − 1.2 V versus Ag/AgCl, KClsat for 180 s. As shown in Fig. 5C, D, the peak current intensity increases with both studied parameters. A compromise between the maximum value and the value where the analysis is sensitive enough and takes a reasonable time leads to choosing as operational parameters the frequency of 10 Hz and the amplitude of 20 mV, respectively.
In order to evaluate which of the two parameters, namely time of deposition and potential of deposition has a more important influence on the peak current intensity for the detection of Cd2+, a single factor ANOVA test was performed, and applied to the values of peak current intensities. The obtained results are summarized in Table 3.
One-factor ANOVA applied to the peak current intensity values for Cd2+ detection showed a significant difference between the obtained results (F > Fcrit, P < 0.05) influenced by the two studied parameters. This means that deposition time and potential have an important influence on Cd2+ deposition. This expected behaviour could be explained by the difference between the physical meaning of time and deposition potential, respectively.
Analytical parameters
The ability of the CPE-15%ADI modified electrode for individual heavy metal ions detection was tested for different concentrations of Zn2+, Cd2+ and Cu2+ ions, by the SWASV—a very sensitive technique—under the optimal operational parameters previously discussed (Fig. 6A).
SWASVs for individual Zn2+, Cd2+ and Cu2+ ions determination at the CPE-15%ADI electrode (A) and the corresponding calibration curve (B). Experimental conditions: electrolyte, 0,1 M acetate buffer (pH 4.5); frequency, 10 Hz; amplitude, 20 mV; step potential 3 mV; deposition time, 180 s; deposition potential, − 1.2 V versus Ag/AgCl, KClsat, starting potential, − 1.2 V versus Ag/AgCl, KClsat.
The calibration curves, presented in Fig. 6B show the linear dependence of the peak current of the metallic ions with the metallic ion concentrations in the range of 1–12 μM, according to the following equation of regression:
The detection limit, calculated for the signal/noise of 3, using the formula LOD = 3 sa/slope (where: sa is the standard deviation of the intercept of the regression equation) led to the following values: 0.48 μM Cu2+ (30.49 μg Cu2+/l), 0.53 μM Cd2+ (59.57 μg Cd2+/l), and 3.87 μM Zn2+ (253.02 μg Zn2+/l), respectively. The obtained limits of detection are lower than those recommended by WHO, i.e., 2000 μg Cu2+/l, 3 μg Cd2+/l, or 3000 μg Zn2+/l [6].
Also, a comparison with the LOD reported in the literature for the use of carbon paste-modified electrodes to determine individually each heavy metal ion (Table 4), led to the conclusion that the newly acquired CPE-15%ADI-modified electrode can be used for Zn2+ and Cu2+ detection with reason-able accuracy at low concentrations. In the case of Cd2+ ions, the detection could be performed only in very concentrated wastewaters which contain large amounts of Cd2+ from accidental spills.
Several methodologies have been developed to enhance the efficiency of materials used for detecting heavy metals. Notably, Raber et al. [44] have explored various pretreatment techniques to modify adsorbents, including chemical activation, thermal treatment, and physical alterations. These pretreatments aimed to increase the surface area, modify pore structures, or introduce functional groups that improve selectivity and binding affinity for specific metal ions.
Contrasting these complex pretreatment methods, our study utilizes untreated clay, leveraging its natural adsorptive properties without any modifications. This approach simplifies the manufacturing process, reduces the overall cost, and minimizes the environmental footprint associated with the production of modifying agents. Due to its inherent properties, untreated clay presents a viable option for sustainable environmental efforts, offering a straightforward and effective solution for heavy metal ion detection.
Reproducibility, term stability and reusability of CPE-15%ADI
Reproducibility represents an essential feature of the modified electrodes involved in electroanalytical detection. As an example, the reproducibility was determined by comparing the relative standard deviations of peak potentials and peak current intensities for four distinct modified electrodes in the presence of 7 μM Cu2+.
Table 5 shows that peak current intensity is more reproducible than the peak potential values.
Term stability of CP-15%ADI electrode material was studied by evaluating, immediately, one day, one week and one month after the preparation, the SWASV signals (in similar investigational conditions as those indicated in Fig. 6) at CPE-15%ADI modified electrodes in the presence of 7 μM Cu2+ in 0.1 M acetate buffer solutions. The results, estimated as the average of peak current, showed a minor diminution in time: (i) 0.02% after one day, 1% after one week, and 2% after one month.
The reusability (operational stability) of the CPE-15%ADI modified electrodes was evaluated by successive SWASV measurements, performed in the presence of 7 μM Cu2+. The experimental conditions are those mentioned in Fig. 6. After 3 consecutive investigations the recorded signal decreased to ~ 97.8%, while after 7 consecutive investigations, it attained ~ 95.5%. The measured performance, combined with the adequate inter-electrode repeatability, suggests that the modified electrode produced is a promising device for heavy metals traces detection.
Real sample analysis
In order to verify the performance of the proposed CPE-15%ADI modified electrode for the individual detection of Zn2+, Cd2+, and Cu2+ in real samples, square wave voltammetry measurements were performed. Thus, drilling water samples were used without spiking, and the determination of metallic ions was carried out using the standard addition method consisting of the addition of different volumes of metallic ions standard solutions (i.e., 2 × 10−3 M) in the real sample (an example for Cd2+ determination is presented in Fig. 7A). The unknown concentration of metallic ions present in the sample was determined by plotting the standard addition calibration curve between current (Ipa/A) against the added concentrations of the standard (cstd/M) (Fig. 7B), by extrapolation of the straight lines of the standard addition calibration to 0Y = 0. The results obtained for 3 replications are summarised in Table 6.
Square-wave anodic stripping voltammograms at CPE-15%ADI for Cd2+ ions detection in real samples of drilling water from mining region Rosia Poieni (department Alba) (A). Calibration curves by standard addition method (B). Experimental conditions: electrolyte, acetate buffer 0.1 M, pH 4.5; frequency, 10 Hz; amplitude, 20 mV; step potential 3 mV; deposition time, 180 s; deposition potential, − 1.2 V versus Ag/AgCl, KClsat; starting potential, − 1.2 V versus Ag/AgCl, KClsat.
For all ions, between the results obtained by using CPE-15%ADI modified electrodes and those achieved by AAS a good correlation was found (Table 6). AAS represents the standardized technique applied for drinking water quality control by the supplier of the water sample. Moreover, for Cu2+ and Zn2+ the concentrations found are in good agreement with the security limits stated for maximum heavy metals amounts in drinking water, suggested by European Drinking Water Directive (2 mg/L) [3, 4], EPA (1 mg/L) [5], and WHO (2 mg/L) [6].
Conclusions
The CPE-15%ADI modified electrode as raw untreated material is non-toxic than classically mercury electrodes and has the advantages of being simple, cost-effective and independent of the dissolved oxygen present in the working solutions. Thus, in comparison with this work, the already published works are based on more elaborated, difficult, problematic, time- and energy-consuming, and polluted techniques. The real advantage of the developed electrode and its originality consists in the “green” material used for the electrode preparation, which leads to competitive results for heavy metals detection.
Stripping analysis recorded at CPE-15%ADI modified electrodes demonstrates a wide linear dynamic range (1–12 μM) for the measured ions and a relatively low detection limit of 0.48 μM Cu2+ (30.49 μg Cu2+/l) < 0.53 μM Cd2+ (59.57 μg Cd2+/l) < 3.87 μM Zn2+ (253 μg Zn2+/l). Other important advantages of the obtained modified electrode are easy handling, easy method of modification, and low cost, thus recommending their use for the detection of Zn2+, and Cu2+ in real water samples (see WHO, UE or EPA), and Cd2+ in very concentrated water samples which contain large amounts of Cd2+ from accidental spills. Moreover, the obtained results proved that the eco-friendly investigated clay can be used in an alternative miniaturised, portable, and mass-production of very inexpensive, simple, fast, and reproducible screen-printed electrodes.
Data and code availability
All relevant data is included in the paper. However, the experimental data are available on request.
References
Waqar A, Hua Z, Junaid M, Mao K, Xu N, Chang C, Rasool A, Aslam MW, Ali J, Yang Z (2021) Insights into the mechanisms of arsenic-selenium interactions and the associated toxicity in plants, animals, and humans: a critical review. Crit Rev Environ Sci Technol 51:704–750. https://doi.org/10.1080/10643389.2020.1740042
Fort CI, Pop LC (2020) Heavy metal and metalloid electrochemical detection by composite nanostructures. In: Baia L, Pap Z, Hernadi K, Baia M (eds) Advanced nanostructures for environmental health. Elsevier, Amsterdam, pp 185–250. https://doi.org/10.1016/b978-0-12-815882-1.000
***, European Drinking Water Directive, Council Directive 92008/105/EC of 16 December 2008 on Environmental Quality Standards in the Eld of Water Policy, E. Union (Ed.), vol. OJ L 348/84 24.12.2008, (Chapter Annex 1).
***, Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast) (Text with EEA relevance) OJ L 435, 23.12.2020, p. 1–62.
***, U.S. Environmental Protection Agency, Risk Assessment, Management and Communication of Drinking Water Contamination; US EPA 625/4-89/024, EPA, Washington, DC. 1989.
***, Guidelines for Drinking-Water Quality, 4th edition, World Health Organization. 2011.
Guo C, Lv L, Liu Y, Ji M, Zang E, Liu Q, Zhang M, Li M (2023) Applied analytical methods for detecting heavy metals in medicinal plants. Crit Rev Anal Chem 53:339–359. https://doi.org/10.1080/10408347.2021.1953371
Rusu MM, Fort CI, Cotet LC, Vulpoi A, Todea M, Turdean GL, Danciu V, Popescu IC, Baia L (2018) Insights into the morphological and structural particularities of highly sensitive porous bismuth-carbon nanocomposites based electrochemical sensors. Sens Actuators B Chem 268:398–410. https://doi.org/10.1016/j.snb.2018.04.103
Fort CI, Cotet LC, Vulpoi A, Turdean GL, Danciu V, Baia L, Popescu IC (2015) Bismuth doped carbon xerogel nanocomposite incorporated in chitosan matrix for ultrasensitive voltammetric detection of Pb (II) and Cd (II). Sens Actuators B Chem 220:712–719. https://doi.org/10.1016/j.snb.2015.05.124
Bansod B, Kumar T, Thakur R, Rana S, Singh I (2017) A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94:443–455. https://doi.org/10.1016/j.bios.2017.03.031
Rodrigues JA, Rodrigues CM, Almeida PJ, Valente IM, Gonçalves LM, Compton RG, Barros AA (2011) Increased sensitivity of anodic stripping voltammetry at the hanging mercury drop electrode by ultracathodic deposition. Anal Chim Acta 701(2):152–156. https://doi.org/10.1016/j.aca.2011.05.031
Ngoc Bui M-P, Li CA, Han KN, Pham X-H, Seong GH (2012) Simultaneous detection of ultratrace lead and copper with gold nanoparticles patterned on carbon nanotube thin film. Analyst 137(8):1888–1894. https://doi.org/10.1039/c2an16020j
Wei Y, Gao C, Meng F-L, Li H-H, Wang L, Liu J-H, Huang X-J (2011) SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium (II), lead (II), copper (II), and Mercury (II): an interesting favorable mutual interference. Phys Chem C 116(1):1034–1041. https://doi.org/10.1021/jp209805c
Romih T, Hočevar SB, Kononenko V, Drobne D (2017) The application of bismuth film electrode for measuring Zn (II) under less acidic conditions in the presence of cell culture medium and ZnO nanoparticles. Sens Actuators B Chem 238:1277–1282. https://doi.org/10.1016/j.snb.2016.09.090
Kadara RO, Tothill IE (2008) Development of disposable bulk-modified screen-printed electrode based on bismuth oxide for stripping chronopotentiometric analysis of lead (II) and cadmium (II) in soil and water samples. Anal Chim Acta 623(1):76–81. https://doi.org/10.1016/j.aca.2008.06.010
Fort CI, Rusu MM, Cotet LC, Vulpoi A, Florea I, Tuseau-Nenez S, Baia M, Baibarac M, Baia L (2021) Carbon xerogel nanostructures with integrated Bi and Fe components for hydrogen peroxide and heavy metal detection. Molecules 26:117. https://doi.org/10.3390/molecules26010117
Etienne M, Cortot J, Walcarius A (2007) Preconcentration electroanalysis at surfactant-templated thiol-functionalized silica thin films. Electroanalysis 19(2–3):129–138. https://doi.org/10.1002/elan.200603677
Guenang LS, Dongmo LM, Jiokeng SLZ, Kamdem AT, Doungmo G, Tonlé IK, Bassetto VC, Jović M, Lesch A, Girault H (2020) Montmorillonite clay-modified disposable ink-jet-printed graphene electrode as a sensitive voltammetric sensor for the determination of cadmium (II) and lead (II). SN Applied Sciences 2(3):476. https://doi.org/10.1007/s42452-020-2283-5
Adams RN (1958) Carbon paste electrodes. Anal Chem 30(9):1576–1576. https://doi.org/10.1021/ac60141a600
Macha SM, Fitch A (1998) Clays as architectural units at modified-electrodes. Mikrochim Acta 128:1–18. https://doi.org/10.1007/BF01242184
Tan X, Liu F, Hu L, Reed AH, Furukawa Y, Zhang G (2017) Evaluation of the particle sizes of four clay minerals. Appl Clay Sci 135:313–324. https://doi.org/10.1016/j.clay.2016.10.012
Ourari A, Tennah F, Ruíz-Rosas R, Aggoun D, Morallón E (2018) Bentonite modified carbon paste electrode as a selective electrochemical sensor for the detection of cadmium and lead in aqueous solution. Int J Electrochem Sci 13:1683–1699. https://doi.org/10.20964/2018.02.35
Srinivasan R (2011) Advances in application of natural clay and its composites in removal of biological, organic, and inorganic contaminants from drinking water. Adv Mater Sci Eng 1–17:872531. https://doi.org/10.1155/2011/872531
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci 140(2):114–131. https://doi.org/10.1016/j.cis.2007.12.008
Jaber L, AbdelHamid AA, Elgamouz A, Kawde A-N (2023) Prediction of a natural clay membrane selectivity towards heavy metal cations removal from wastewater: an electrochemical study. J Solid State Electrochem. https://doi.org/10.1007/s10008-023-05774-6
El-kordy A, Nizar S, Tijani N, Kawde A-N, Elgamouz A (2024) A comparative study of treated clay and synthesized Fe/ZSM-5 zeolite for application in the electrochemical reduction of chromium(VI) as an environmental sensor. J Solid State Electrochem. https://doi.org/10.1007/s10008-024-05877-8
El-Kordy A, Elgamouz A, Abdelhamid A, Kawde A-N, Tijani N, Lemdek EM (2024) Manufacturing of novel zeolite-clay composite membrane from natural clay and diatomite, an electrochemical study of the surface and application towards heavy metals removal. J Environ Chem Eng 12(2):112143. https://doi.org/10.1016/j.jece.2024.112143
Navratilova Z, Kula P (2003) Clay modified electrodes: present applications and prospects. Electroanalysis 15(10):837–846. https://doi.org/10.1002/elan.200390103
Lazaroiu G, Frentiu T, Mihaescu L, Mihaltan A, Ponta M, Frentiu M, Cordos E (2009) The synergistic effect in coal/biomass blend briquettes combustion on elements behavior in bottom ash using ICP-OES. J Optoelectron Adv M 11:713–721
Ilander A, Vaisanen A (2011) The determination of antimony and arsenic concentrations in fly ash by hydride generation inductively coupled plasma optical emission spectrometry. Anal Chim Acta 689:178–183. https://doi.org/10.1016/j.aca.2011.01.043
Scrob T, Covaci E, Hosu A, Tanaselia C, Casoni D, Torok AI, Frentiu T, Cimpoiu C (2022) Effect of in vitro simulated gastrointestinal digestion on some nutritional characteristics of several dried fruits. Food Chem 385:132713. https://doi.org/10.1016/j.foodchem.2022.132713
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry, Pearson Education Ltd, Edinburgh Gate, England
Méité N, Konan LK, Tognonvi MT, Goure Doubi BIH, Gomina M, Oyetola S (2021) Properties of hydric and biodegradability of cassava starch-based bioplastics reinforced with thermally modified kaolin. Carbohydr Polym 254:117322. https://doi.org/10.1016/j.carbpol.2020.117322
Soro SB, Coulibaly M, Gauly LP, N’Dri SR, Sanou A, Trokourey A (2023) Characterization of clay materials from Côte d’Ivoire: possible application for the electrochemical analysis. J Mat Sci Res 12:51–64. https://doi.org/10.5539/jmsr.v12n1p51
Kouakou LPM-S, Karidioula D, Manouan MRW, Pohan AGL, Cissé G, Konan LK, Andji-Yapi JY (2023) Use of two clays from Côte d’Ivoire for the adsorption of methyl red from aqueous medium. Chem Phys Letters 810:140183. https://doi.org/10.1016/j.cplett.2022.140183
Gómez Y, Fernández L, Borrás C, Mostany J, Scharifker B (2011) Characterization of a carbon paste electrode modified with tripolyphosphate-modified kaolinite clay for the detection of lead. Talanta 85:1357–1363. https://doi.org/10.1016/j.talanta.2011.06.014
Shetti NP, Nayak DS, Kuchinad GT, Naik RR (2018) Electrochemical behavior of thiosalicylic acid at γ-Fe2O3 nanoparticles and clay composite carbon electrode. Electrochim Acta 269:204–211. https://doi.org/10.1016/j.electacta.2018.02.170
Dao K, Ouedraogo M, Millogo Y, Aubert J-E, Gomina M (2018) Thermal, hydric and mechanical behaviours of adobes stabilized with cement. Constr Build Mater 158:84–96. https://doi.org/10.1016/j.conbuildmat.2017.10.001
Akanji SP, Arotiba OA, Nkosi D (2019) Voltammetric determination of Pb (II) ions at a modified kaolinite-carbon paste electrode. Electrocatalysis 10:643–652. https://doi.org/10.1007/s12678-019-00552-3
Brett CMA, Brett AMO (1993) Electrochemistry: principles, methods, and applications. Oxford University Press, Oxford, pp 176–190
Ye J-S, Wen Y, Zhang WD, Cui H-F, Gan LM, Xu GQ, Sheu F-S (2004) Application of multi-walled carbon nanotubes functionalized with hemin for oxygen detection in neutral solution. J Electroanal Chem 562:241–246. https://doi.org/10.1016/j.jelechem.2003.09.007
Yan Q, Zhi N, Yang L, Xu G, Feng Q, Zhang Q, Sun S (2020) A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci Reports 10:10607. https://doi.org/10.1038/s41598-020-67394-8
Hermouche L, Aqil Y, Abbi K, El Hamdouni Y, Ouanji F, El Hajjaji S, El Mahi M, Lotfi EM, Labjar N (2021) Eco-friendly modified carbon paste electrode by Bigarreau Burlat kernel shells for simultaneous trace detection of cadmium, lead, and copper. Chem Data Coll 32:100642. https://doi.org/10.1016/j.cdc.2020.100642
Raber G, Kalcher K, Stadlober M (1997) New voltammetric methods for the determination of heavy metals using a montmorillonite modified carbon paste electrode. Sci Pap Univ Pardubice Ser A 3:163–193
Altahan MF, Ali AG, Hathoot AA, Abdel-Azzem M (2021) NaOH pretreated molybdate-carbon paste electrode for the determination of phosphate in seawater by square wave voltammetry with impedimetric evaluation. J Electrochem Soc 168:127503. https://doi.org/10.1149/1945-7111/ac3b03
Devnani H, Rajawat DS, Satsangee SP (2014) Black rice modified carbon paste electrode for the voltammetric determination of Pb (II), Cd (II), Cu (II) and Zn (II). Proc Nat Acad Sci India Sect A Phys Sci 84:361–370. https://doi.org/10.1007/s4001
Rajawat DS, Kardam A, Srivastava S, Satsangee SP (2013) Nanocellulosic fiber-modified carbon paste electrode for ultra trace determination of Cd (II) and Pb (II) in aqueous solution. Environ Sci Pollut Res Int 20:3068–3076. https://doi.org/10.1007/s11356-012-1194-4
Sahoo PK, Panigrahy B, Sahoo S, Satpati AK, Li D, Bahadur D (2013) In situ synthesis and properties of reduced graphene oxide/Bi nanocomposites: as an electroactive material for analysis of heavy metals. Biosens Bioelectron 43:293–296. https://doi.org/10.1016/j.bios.2012.12.031
Flores-Álvarez JM, Cortés-Arriagada D, Reyes-Gómez J, Gómez-Sandoval Z, Rojas-Montes JC, Pineda-Urbina K (2021) 2-Mercaptobenzothiazole modified carbon paste electrode as a novel copper sensor: An electrochemical and computational study. J Electroanal Chem 888:115208. https://doi.org/10.1016/j.jelechem.2021.115208
Acknowledgements
Ali Sanou acknowledges the financial support by Eugen Ionescu fellowship 2020-2021 offered by Agence Universitaire de la Francophonie (AUF) and The Romanian Government. The authors thank dr. Lucian Barbu-Tudoran (National Institute for Research and Development of Isotopic and Molecular Technologies, 65-103 Donath St., RO-400293 Cluj-Napoca, Romania) for SEM images and dr. Adrian Ionut Cadis, and dr. Laura Elena Muresan (Raluca Ripan Institute for Research in Chemistry, Babes-Bolyai University, 30 Fantanele St., RO- 400294 Cluj-Napoca, Romania) for FtIR spectrum.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.S., M.C., P.J.M.R.D.; C.I.F., and G.L.T.; methodology, A.S., C.I.F., and G.L.T.; software, A.S., C.I.F., and G.L.T.; validation, S.R.N, C.I.F., and G.L.T.; formal analysis, S.R.N, K.D.M.A., C.I.F., and G.L.T.; investigation, A.S., T.L.T., L.B., T.F., and E.C.; data curation, A.S., C.I.F., and G.L.T.; writing—original draft preparation, A.S.; T.L.T., L.B., T.F., E.C., C.I.F., and G.L.T.; writing—review and editing, A.S., M.C., S.R.N; K.D.M.A.; T.L.T., L.B., C.I.F., and G.L.T.; visualization, A.S., M.C., S.R.N.; T.L.T., L.B., T.F., E.C., K.D.M.A., K.B.Y., C.I.F., and G.L.T.; supervision, M.C., P.J.M.R.D.; K.B.Y., C.I.F., and G.L.T.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service or company that could be construed as influencing the position presented in the manuscript.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanou, A., Coulibaly, M., N’dri, S.R. et al. Raw clay material-based modified carbon paste electrodes for sensitive heavy metal detection in drinking water. J Mater Sci (2024). https://doi.org/10.1007/s10853-024-09945-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10853-024-09945-2