Abstract
This work focuses on the superconducting and magnetic properties of Chevrel phase (CP) compounds (Cu2Mo6S8 and Ni2Mo6S8) processed via a novel self-propagating high-temperature synthesis (SHS) method involving ternary cation intercalation in MoS2 high-temperature polymorphs. Green pellets of the precursor materials sealed under vacuum and exposed to 1050 °C or 1200 °C rapidly transformed from a powder mixture into submicron CPs within minutes. X-ray diffraction analysis confirmed the presence of Cu2Mo6S8 CP (67%), and Ni2Mo6S8 (93%). This group has illustrated the first time CP were formed at low energy cost and full stoichiometry. Magnetization versus temperature data obtained for the samples under different field conditions confirmed the superconducting behavior of the Cu–CP at a critical temperature of 8.3 K. The Ni–CP behaved as a superparamagnetic at low temperature (4.2 K). The results of this work suggest that the novel SHS process offers a viable scalable synthesis route for the Chevrel family of compounds and that it is worth exploring the functional applications of the new compounds of this material system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chevrel phase (CP) compounds were discovered in 1973 when Dr. Marcel Sergent and Roger Chevrel synthesized compounds via solid state synthesis at elevated temperatures inside of a fused silica tube using MoS2 and a third element (M) [1]. The formula of the CP family of compounds is illustrated as MxMo6Z8, where M is a ternary cation, and Z is either sulfur, selenium, or tellurium; x may either fall between zero and two for bivalent M or between zero and four for monovalent M. The unique structure of CP (discussed in detail below) allows for the insertion of over 40 different elements as ternary cations, which can be used to tune the specific properties of the compound for different applications leading to a multifunctional material. Due to this flexibility, CP compounds have been investigated for applications as thermoelectrics [2, 3], battery electrodes [4, 5], electrocatalysts [6, 7], superconductors [8, 9], and others. Although superconductivity in CP has been observed before, superconducting compounds had not been synthesized via self-propagating high-temperature synthesis (SHS), which has the potential for industrial scalability over other methods of CP synthesis. Therefore, it is critical to test SHS viability if CP compounds are to be pursued for applications in the superconductivity industry.
Self-propagating high-temperature synthesis refers to a reaction in which precursor materials are ignited and spontaneously transformed into the product due to the reaction’s highly exothermic nature [10]. Specifically, a combustion front propagates through the reactants with a certain velocity to transform the reactants into the product [11]. A novel SHS process was illustrated in Gouma’s previous work, which involved a multi-step reaction mechanism for the rapid and sustainable processing of 3D and 2D ternary chalcogenides [12]. This reaction mechanism is attributed to a synergetic action of polymorphic, intercalation, and exfoliation processes taking place via the following stages: (1) MoS2 phase transformation; (2) Ternary cation intercalation; (3) Exfoliation; (4) CP formation [13].
SHS was employed for this experiment to demonstrate a low energy method of synthesizing CP compounds. Other methods of synthesis include solid state [1, 14, and 15], molten salt [16], solution precipitation [17], high energy mechanical milling [18], and microwave [19]. While, there are many options available for synthesizing CP, these methods include drawbacks that would prove difficult in scalability. For example, solid state, molten salt, and solution precipitation syntheses all require lengthened furnace times of up to 60 h [16, 17, 20, 21], which can cost large amounts of energy. Furthermore, while high energy mechanical milling and microwave synthesis do not require these extended heating times, they do suffer from consistent incomplete phase transitions [18] and the need for more complex and/or expensive equipment (that has its own difficulties in upscaling) [19] that reduce their viability for scaling to industrial levels. Due to these factors, SHS is the most promising method for scalability as it uses a simple mechanism of mixing commercially available dry powders, pressing, vacuuming, and heat treating; it requires reduced heat treatment times leading to lower energy inputs; and it has shown viability in creation of fully transformed samples [12].
For this specific SHS route, MoS2 was chosen as a precursor over just elemental Mo and S to allow for an intercalation-based synthesis method. Bulk MoS2 has three major polytypes with 1T (trigonal), 2H (hexagonal), and 3R (rhombohedral) symmetry. The 2H phase is thermodynamically stable at room temperature and the metastable phases arise from highly non-equilibrium thermodynamic conditions. Transition metals can easily intercalate through the Van der Waals gaps in MoS2. This process usually involves electron transfer from the guest intercalate to the higher energy unoccupied d band of molybdenum. The negatively charged S layer is considered to be a driving force for the coulombic attraction between the intercalates and Mo. The steps involved in the intercalation process include adsorption of ternary cations (intercalate) on the surface of the MoS2 crystal, formation of an activated complex, followed by rapid diffusion around the edge, with subsequent entry into the Van der Waals gaps of MoS2. For example, intercalation of Fe in MoS2 forms the layered compound FeMo2S4. In Gouma’s earlier work, formation of FeMo3S4, hypothesized through intercalation of Fe through MoS2, was observed within just 1 min of residence time in the SHS chamber [13]. This intercalation-based SHS process allows for the rapid synthesis of CP compounds from a low energy method that could be highly desirable for commercial applications.
Experimental method
Material synthesis via SHS
Powders of Cu (99.5% 0.8 µm, US Research Nanomaterials), Ni (99.9% 1–1.5 μm, SkySpring Materials), Mo (99.9% 0.5–0.8 µm, SkySpring Materials), and MoS2 (Super Fine Grade, Climax Molybdenum) were used in synthesizing the CPs. Powders with a ratio of 4Cu:2Mo:4MoS2 to a total weight of 10 g were vigorously mixed using a Janke and Kunkel laboratory grinder. 2 g of the mixture was then measured out and mechanically pressed into a cylindrical pellet of 0.5-inch diameter with 15000lbs of force. A novel reaction chamber using a quartz tube and a vacuum adapter was employed to encapsulate the pellet in vacuum in an effort to remove oxygen and moisture from the reaction environment (see Fig. 1 below). Additionally, alumina wool was added into the reaction chamber to assist with thermal isolation.
The encapsulated 4Cu:2Mo:4MoS2 sample in the reaction chamber was introduced into a general–purpose tube furnace (Thermo Scientific Lindberg) at 1050 °C for 20 min before the reaction chamber was removed and allowed to cool to room temperature. Once cooled, the vacuum was released under a fume hood to avoid vapor exposure. The same process of grinding, pressing, and encapsulating was used for a ratio of 2Ni:2Mo:4MoS2. The Ni sample was heat-treated at 1200 °C for 10 min before removal.
XRD
Portions of the heat-treated samples were ground into a fine powder using a mortar and pestle for powder X-ray diffraction (XRD). XRD was carried out at the center for electron microscopy and analysis (CEMAS) using a Rigaku SmartLab X-ray diffractometer (Bragg–Brentano unit). The tube voltage and current were set to 40 kV and 15 mA, respectively. An angle range of 10°–60° with a step size of two per min was used for 2θ values. Resulting spectra were analyzed using MDI JADE and the ICDD database.
SEM
Powder samples were also observed via scanning electron microscopy (SEM) to analyze morphology and crystallite size. Processed samples were imaged using the Thermo Scientific Apreo II SEM and the Thermo Scientific Quattro environmental scanning electron microscope (ESEM) at CEMAS. Image analysis was completed using ImageJ.
SQUID magnetometer
In order to fit within the ~ 5 mm homogeneous region of the superconducting quantum interference device (SQUID) magnetometer, each pellet was fractured, and a piece of suitable size was attached to the inside of a plastic drinking straw using GE 7031 varnish. The straw was cut so the sample was approximately 66 mm from the holder end, then attached to a sample holder rod and lowered into a Quantum Design MPMS 3 SQUID magnetometer. The temperature was set to 2 K with zero applied field (zero-field-cooled condition, or ZFC), after which a 5 m T field was applied and the sample was centered by sensing the moment as a function of vertical position. Measurements of magnetic moment were performed in Vibrating-Sample magnetometer (VSM) mode with an amplitude of 5 mm and frequency of 11.7 Hz. The magnetic moment was measured versus temperature, sweeping from 1.8 K to 10 or 20 K at a rate of 3 K/min. The sample was cooled under applied field (field-cooled or FC) and then moment versus increasing temperature was measured again. The field was then reset to zero. This sequence was repeated for applied magnetic fields of 1 T for the Cu sample and 1, 3, 5, and 7 T for the Ni sample. A magnetization versus field (M vs. H) measurement was performed, where the field was swept from zero, to a positive value, then to a negative value, and then to positive (five quadrants) at a rate of 10 m T/s. Moment versus temperature was then measured up to 313 K at a faster sweep rate. An M versus H measurement was also conducted at 300 K. The susceptibility was obtained by dividing the measured moment by the sample volume.
Results
XRD
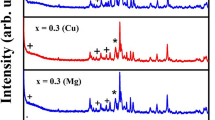
Figure 2 below illustrates the XRD patterns for the 4Cu:2Mo:4MoS2 sample (Fig. 2a) and 2Ni:2Mo:4MoS2 sample (Fig. 2b). As can be seen, the 4Cu:2Mo:4MoS2 sample was mostly transformed into the Cu2Mo6S8 CP with a presence of MoS2. Additionally, in this sample was a presence of Cu2O due to a side reaction during the heat treatment process (discussed later). For the 2Ni:2Mo:4MoS2 sample (Fig. 2b), Ni2Mo6S8 CP is the prominent phase with some presence of MoS2. No additional Ni peak was present in the scan as during heat treatment, some stray Ni powder adhered to the quartz tube enclosure.
Figure 3 displays the wt% of each phase as calculated by JADE via peak intensities for each sample. As shown, the CP for the Ni sample is much more prominent than the respective CP of the Cu sample. Further investigation is ongoing to determine if cation particle size and/or time is a main contributor to this difference.
SEM
ImageJ was used to analyze the crystallite size of the CPs as well as the MoS2 platelets in the 2Ni:2Mo:4MoS2 sample. Figure 4 below exhibits the morphology of the 4Cu:2Mo:4MoS2 sample. The morphology is uniform with faceted crystals of a size ranging between 700 nm and 2.2 μm.
Figure 5 illustrates the morphology of the 2Ni:2Mo:4MoS2 sample. There are large Ni CP crystals in the range of 900 nm–2.2 μm along with MoS2 platelets in the range of 1.2 um–3.6 μm. Also, observed is a large amount of sintering and coarsening, suggesting that the heat treatment time of 10 min is rather long for this particular reaction.
SQUID magnetometer
The M versus T data indicates superconductivity in both samples. At low temperature, the sample cooled in zero-field conditions (ZFC) exhibited shielding currents once field was applied due to the Meissner effect which opposed the applied magnetic field, giving rise to a magnetization. However, in the field cooled condition, the Meissner effect would be expected to cause flux expulsion below Tc; the fact that the magnetization remains near zero is indicative of significant flux pinning.
The critical temperature Tc was chosen as the temperature above which the gap between the zero-field-cooled and field-cooled curves went to zero, in the case of the Cu CP, or stopped rapidly converging, in the case of the Ni CP. The modified definition was necessary in the case of the Ni sample because above Tc the sample is paramagnetic and there remains a gap between the ZFC and FC curves. See, Table 1 below for Tc values of the two samples.
The M versus T data for the 4Cu:2Mo:4MoS2 sample (Fig. 6) shows that the material is superconducting up to 8.3 K in 5 m T applied field and 7.0 K in 1 T field. The shape of the M versus H loop (Fig. 7) is also indicative of a type II superconductor with flux pinning. The magnetization increases with decreasing magnitude of the applied field, but then goes rapidly to near zero in the range ± 0.2 T, a behavior consistent with flux jumps and likely due in part to a large size of the superconducting regions (the tendency to flux jump is proportional to the critical current density times the sample size). While, a single flux jump was recorded in each pass through the low field in this case, measurements on similar Cu CP samples showed multiple flux jumps.
Figure 8 below illustrates the susceptibility versus T measurement for the 2Ni:2Mo:4MoS2 sample and shows a slight difference between the ZFC and FC curves at 5 m T below ~ 3.4 K, consistent with a superconducting transition, though this ΔM is orders of magnitude smaller than in the Cu sample. In Fig. 9 the M versus H measurement shows behavior which might indicate a super paramagnet or spin glass at 4.2 K. At 300 K, the sample is paramagnetic with a ferromagnetic component that saturates around 0.1 T, which may be present in the 4.2 K data as well. The susceptibility versus T data above Tc, at 5 m T, is well within the range that the ferromagnetic component would dominate.
Figure 10 below illustrates the M versus H data for both samples at 300 K.
Discussion
Chevrel structure
The multifunctionality of the CP family stems from the unique building blocks of Mo6Z8, in which Mo atoms form an octahedron with each atom placed slightly outside of a surrounding distorted cube of Z atoms (see Fig. 11a). Each corner of each Mo6Z8 cube lies opposite of the face center of the adjacent cubes, leading to close contacts of the six Mo atoms of one unit and the Z atoms of the surrounding six units. In the full structure, each Mo atom in a unit is located close to five Z atoms, creating a square base pyramid. The four Z atom base of such a pyramid constitutes the face of a single Mo6Z8 unit, and the fifth apex Z atom is a part of an opposite Mo6Z8 unit. Therefore, the 6f Z atoms in a building block belong to bases of the pyramids and are simultaneously the apexes of six pyramids, while the remaining 2c Z atoms belong only to the square faces of the pyramids [6].
This layout provides different cavities in the chalcogenide network. The largest (Site 1 in Fig. 11b) is at the origin of the rhombohedral unit cell and creates a distorted cube with eight atoms from eight different Mo6Z8 units. The second and third cavities are much smaller; the second cavity (Site 2) is located between two of Site 1, and the third cavity (Site 3) is located between two consecutively misaligned Mo6Z8 units [6]. Ternary cations will fill specific cavities depending on their size and create channels of ternary cations due to the interconnected network. This network allows for the insertion of over 40 elements as ternary cations. Figure 12 illustrates a model of the CP structure of the formula M4Mo6S8.
Naturally, having different sites in the Mo6S8 can result in different CP structures. Singstock et al. [22] used machine learning to predict the placement of over 50 elements in the Chevrel structures for Mo6S8, Mo6Se8, and Mo6Te8. According to Fig. 13a below, for Mo6S8 the Cu atoms have a near even affinity towards Site 1 center and Site 1 off-center (Site 2 in Fig. 11) with minimal chance of occupying Site 2 (Site 3 in Fig. 11). Conversely, Ni shows a greater affinity towards Site 1 off-center and a less chance to occupy Site 1 center or Site 2. From this analysis larger cations prefer Site 1, while smaller cations prefer Site 1 off-center. In Mo6S8, Site 2 is predicted to be reserved mainly for 4D and 5D transition metal intercalants. In addition to changes in stability for an element in varying sites, Singstock also predicted that changes in the electronic structure may occur. For example, some CPs may transition from metallic to semiconducting or from nonmagnetic to antiferromagnetic as the ternary cation site changes.
Cation prediction sites for different Chevrel structures. a Mo6S8. b Mo6Se8. c Mo6Te8. Reprinted with permission from [22] copyright 2024 American chemical society.
Within this study, the unique electronic and magnetic properties of Cu and Ni in the cluster compounds was shown, but further work is needed to establish the specific property effects of Cu and Ni on the clusters.
SHS process
After the synthesis, the CP present for the 4Cu:2Mo:4MoS2 sample was found to be Cu2Mo6S8. This phase (from [23]) was confirmed to most closely match the experimental XRD peaks, which was also compared to reference scans for Cu2Mo3S4 [24], Cu1.73Mo6S7.9, Cu0.67Mo2S2.53 [25], CuMo6S8 [26], as well as a few other copper–deficient stoichiometries. These other scans displayed peak variations from the 4Cu:2Mo:4MoS2 experimental sample especially around 2θ = 45°. E.g., at this degree there are two prominent peaks shown in the experimental sample and the Cu2Mo6S8 reference (at 45.70° and 46.20°), but for Cu2Mo3S4 this is one large peak (45.70°) and for Cu1.73Mo6S7.9 (at 45.20°, 45.60°, and 46.40°), this is three smaller peaks. Cu0.67Mo2S2.53 contains the closest peak position to the experimental, however the relative peak intensities are off compared to the closer matched Cu2Mo6S8 phase.
Similarly, the 2Ni:2Mo:4MoS2 sample was compared to reference XRD scans for Ni0.7Mo3S4 [27], NiMo3S4 [28], Ni2.5Mo6S6.7 [29], and Ni2Mo6S8 [30]. Ni2.5Mo6S6.7 shows two very small peaks around 2θ = 33.30° and 32.60° that do not appear in the experimental sample, and Ni0.7Mo3S4 showed a small peak shift that was not seen in the sample. Although NiMo3S4 and Ni2Mo6S8 are the same stoichiometrically, there exists a very small difference in a peak at 2θ = 45.70°, where in NiMo3S4 a second peak begins to emerge out of the side at 45.90°. This second peak is not seen in Ni2Mo6S8 or in the experimental sample leading to the conclusion that Ni2Mo6S8 is the CP present.
While, the samples were not transformed into 100% CP, the SHS process was shown to be viable for CP formation. One noted result was the presence of Cu2O in the 4Cu:2Mo:4MoS2 sample. This phase was found to be due to a rather prominent amount of Cu2O contaminant within the Cu powder precursor (See Fig. 14 below). The XRD scan below shows the presence of about 31.1% Cu2O within the powder along with 17.7% Na2SO4. The Cu2O is believed to have caused the low Chevrel yield as the Cu2O could not participate in the intercalation mechanism. The peaks for Na2SO4 are expected to have been minor enough in the final product that they fell within the background of the spectrum. Nb., this copper powder was re-sourced for ongoing experiments and the purity has been confirmed.
Superconducting properties
Copper-based CP (CuxMo6S8) is stable for a range of copper content [10]. Two low temperature phases are reported: LT1 with a critical temperature Tc which increases from 10.0 K at x = 1.75 to 10.9 K at x = 1.85, and LT2, with a Tc which decreases from 6.3 K at x = 3.1 to 4.0 K at x = 3.3 [11]. See below in Table 2 for a list of selected common superconducting materials for comparison. The Tc of 8.3 K in our sample lies between the Tcs of the two LT phases, and no composition is shown in [11] to have that Tc. The reason for the difference in our Tc is unknown at this time, but Tc is known in other materials to be affected by composition and lattice strain. The magnetization versus field loop for the Cu CP shows large hysteresis consistent with flux pinning behavior. The loop widths decrease to near zero but have long tails before finally closing at the irreversibility field Birr, which is > 4 T.
In addition to the superconducting behavior, there also appears to be a paramagnetic component. This background makes determination of the upper critical field, BC2, difficult, since in magnetometry measurements it is commonly determined by the point, where the slope of the M–H loop goes from positive to zero.
While, the low Tc and BC2 rule out these samples for use in applications such as high-field magnets, a Tc above 4.2 K enables use in liquid helium cooled environments without the complexity involved in obtaining lower temperatures and could be useful in electronics, sensors, or other novel applications.
Our susceptibility measurement for the 2Ni:2Mo:4MoS2 sample and showed a slight difference between the ZFC and FC curves at 5 m T below ~ 3.4 K, consistent with a superconducting transition, though this ΔM is orders of magnitude smaller than in the Cu sample. This is interesting, because the Ni-containing compound is one of several CP materials (M = Cr, Mn, Fe, Co, Ni) which is not known to be superconducting because even small amounts of magnetic impurity are enough to destroy superconductivity in most such materials [12]. We also observe superparamgnetic signatures, and an apparent additional soft ferromagnetic behavior. Paramagnetism has been reported previously in Ni containing CP materials using tellurium instead of sulfur [20].
Conclusions
CPs of Cu2Mo6S8 and Ni2Mo6S8 were processed via intercalation-assisted SHS process and were tested for their magnetic and superconducting properties. The 4Cu:2Mo:4MoS2 sample was shown to be superconducting up to 8.3 K in the 5 m T field and decreased to 7.0 K in 1 T applied field. The Cu CP performed approximately within the Tc range of Cu CP with different compositions, synthesized via other methods. Even though the Tc is still relatively low compared to more conventional superconducting materials, it was worth assessing the novel microstructures. The 2Ni:2Mo:4MoS2 sample showed some evidence for a superconducting response below ~ 3.4 K. Also, we saw superparamagnetic behavior at low temperature, which is consistent with other (ternary) Ni chalcogenides. Additionally, the samples were found to have ferromagnetic or paramagnetic properties. This background made the determination of the upper critical field via magnetometry difficult. A Tc above 4.2 K enables use in liquid helium cooled environments without the complexity involved in obtaining lower temperatures. The energy efficient and novel rapid synthesis route demonstrated here to produce compounds of the CP family may open the pathway for the novel configurations and properties of these interesting cluster compounds.
Supplementary information
Repetitive XRD scans were omitted from the manuscript as they were not required to fairly represent the above work.
Data availability
Data files from the XRD scans may be obtained by contacting the corresponding author.
References
Perrin A, Christiane P, Chevrel R (2019) chevrel phases: genesis and developments. In: Halet J-F (ed) Ligated transition metal clusters in solid-state chemistry: the legacy of marcel sergent. Springer, Cham, pp 1–30
Caillat T, Fleurial JP, Snyder GJ (1999) Potential of chevrel phases for thermoelectric applications. Solid State Sci 1(7):535–544. https://doi.org/10.1016/S1293-2558(00)80105-3
Tsubota T, Ohtaki M, Eguchi K (1999) Thermoelectric properties of chevrel-type sulfides AMo6S8 (A=Fe, Ni, Ag, Zn, Sn, Pb, Cu). J Ceram Soc Jpn 107:697–701. https://doi.org/10.2109/jcersj.107.697
Uchida T, Tanjo Y, Wakihara M, Taniguchi M (1990) Nickel-molybdenum sulfide Ni2Mo6S7.9 as the cathode of lithium secondary batteries. J Electrochem Soci. https://doi.org/10.1149/12086443
Aurbach D et al (2007) Progress in rechargeable magnesium battery technology. Adv Mater 19(23):4260–4267. https://doi.org/10.1002/adma.200701495
McCarty KF, Schrader GL (1984) Hydrodesulfurization by reduced molybdenum sulfides: activity and selectivity of chevrel phase catalysts. Ind Eng Chem Prod Res Develop 23:519–524
Xia F et al (2021) Carbon free and noble metal free Ni2Mo6S8 electrocatalyst for selective electrosynthesis of H2O2. Adv Funct Mater 31(47):2104716. https://doi.org/10.1002/adfm.202104716
Flukiger R et al (1977) The low temperature phase diagram of the system CuxMo6S8 in the range 1.5≤×≤3.55. Solid State Commun 23(10):699–702. https://doi.org/10.1016/0038-1098(77)90475-6
Seeber B (2023) Prospects of an alternative superconductor technology for fusion reactors. Phy Plasmas. https://doi.org/10.1063/50162284
Subrahmanyam J, Vijayakumar M (1992) Self-propagating high-temperature synthesis. J Mater Sci 27:6249–6273 https://doi.org/10.1007/BF00576271
Merzhanov AG, Borovinskaya IP (1972) Self-spreading high-temperature synthesis of refractory inorganic compounds. Doklady Akademii Nauk SSSR Seriya Khimiya 204(2):366–369
M. Pawar (2021) Self-propagating high temperature synthesis (SHS) of semi-conducting Chevrel phase compounds, Doctor of Philosophy Materials Science and Engineering. The Ohio State University
Pawar M, Gouma PI (2022) Intercalation-assisted massive phase transformation: the key to SHS synthesis? J Am Ceram Soc 105:515–519. https://doi.org/10.1111/jace.18724
Chevrel R, Sergent M, Prigent J (1971) Sur de nouvelles phases sulfurées ternaires du molybdène. J Solid State Chem 3(4):515–519. https://doi.org/10.1016/0022-4596(71)90095-8
Naik K, Sampath S (2017) Cubic Mo6S8 -efficient electrocatalyst towards hydrogen evolution over wide pH range. Electrochim Acta 252:408–415. https://doi.org/10.1016/j.electacta.2017.09.015
Lancry E, Levi E, Mitelman A, Malovany S, Aurbach D (2006) Molten salt synthesis (MSS) of Cu2Mo6S8—new way for large-scale production of chevrel phases. J Solid State Chem 179(6):1879–1882. https://doi.org/10.1016/j.jssc.2006.02.032
Cheng Y et al (2014) Facile synthesis of chevrel phase nanocubes and their applications for multivalent energy storage. Chem Mater 26(17):4904–4907. https://doi.org/10.1021/cm502306c
Saha P et al (2017) A rapid solid-state synthesis of electrochemically active Chevrel phases (Mo6T8; T = S, Se) for rechargeable magnesium batteries. Nano Res 10(12):4415–4435. https://doi.org/10.1007/s12274-017-1695-z
Murgia F, Antitomaso P, Stievano L, Monconduit L, Berthelot R (2016) Express and low-cost microwave synthesis of the ternary chevrel phase Cu2Mo6S8 for application in rechargeable magnesium batteries. J Solid State Chem France 242:151–154. https://doi.org/10.1016/j.jssc.2016.07.022
Chevrel R, Sergent M (1982) Chemistry and structure of ternary molybdenum chalcogenides. In: Fischer O, Maple MB (eds) Superconductivity in ternary compounds i: structural, electronic, and lattice properties. Springer, Berlin and Heidelberg, pp 25–86
Even-Boudjada S, Burel L, Chevrel R, Sergent M (1998) New synthesis route of PbMo6S8 superconducting chevrel phase from ultrafine precursor mixtures: I. PbS, MoS2 and mo powders. Mater Res Bull 33(2):237–252. https://doi.org/10.1016/S0025-5408(97)00229-8
Singstock NR, Ortiz-Rodríguez JC, Perryman JT, Sutton C, Velázquez JM, Musgrave CB (2021) Machine learning guided synthesis of multinary chevrel phase chalcogenides. J Am Chem Soc 143(24):9113–9122. https://doi.org/10.1021/jacs.1c02971
Umarji AM, Rao GVS, Janawadkar MP, Radhakrishnan TS (1980) Metal atom incorporation studies on AxMo6S8 chevrel phases. J Phy Chem Solids 41(5):421–429. https://doi.org/10.1016/0022-3697(80)90169-9
Potel M (1984) Labilite des cations dans les chalcogenures ternaires de molybdene: voies d’acces a de nouvelles syntheses. Revue de Chimie Minerale 21(4):509–536
Cheung KY, Steele BCH (1980) Homogeneity range of copper molybdenum sulphide CuxMo6S8−y. Mater Res Bull 15(12):1717–1725. https://doi.org/10.1016/0025-5408(80)90189-0
Tovar M, DeLong LE, Johnston DC, Maple MB (1979) Superconductivity and crystal structure of ternary CuxMo3S4 compounds with 0 ≤ x ≤ 1 prepared by anodic oxidation of Cu1.0Mo3S4. Solid State Commun 30(9):551–557. https://doi.org/10.1016/0038-1098(79)91135-9
Guillevic J, Bars O, Grandjean D (1976) Etude structurale de combinaisons sulfurees et seleniees du molybdene v structures cristallines de phases MxMo3S4 (M=Ni, Co, Fe). Acta Crystallographica Sect B 32(5):1338–1342
Guillevic J, Bars MO, Grandjean D (1973) Etude structurale de combinaisons sulfurées et séleniées du molybdène III structure cristalline de NiMo3S4. J Solid State Chem 7(2):158–162. https://doi.org/10.1016/0022-4596(73)90149-7
Chang CL, Tao YK, Swinnea JS, Steinfink H (1987) Oxygen substitution in Sn and Ni Chevrel phases. Acta Crystallographica Sect C 43(8):1461–1465
Ritter C, Nöldeke C, Press W, Stege U, Schöllhorn R (1993) High Ni2+-mobility in the chevrel phase Ni2Mo6S8: a quasielastic neutron scattering study. Z für Phys B Condensed Matter 92(4):437–442. https://doi.org/10.1007/BF01320505
Acknowledgements
The authors would like to acknowledge Dr. Zanlin Qiu for assistance with the Thermo Scientific Apreo II SEM. The authors would also like to acknowledge the Edward Orton Jr. Ceramic Foundation for funding the presented research.
Funding
Edward Orton Jr. Ceramic Foundation
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: David Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gilmore, T., Rochester, J., Sumption, M. et al. Intercalation based synthesis of Chevrel phase superconductors. J Mater Sci 59, 11880–11892 (2024). https://doi.org/10.1007/s10853-024-09910-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09910-z