Abstract
Geopolymers belong to the broad family of alkali-activated materials that are considered to have much smaller carbon dioxide (CO2) footprint than traditional Portland cements. A wide range of metallurgical wastes are utilized as silicious sources to produce geopolymer components with a prospect of numerous applications in the construction field. At the same time, efforts have been also made to the valorization of aplite rock, which is found to be abundant in Finnvolldalen of Norway and consists mainly of quartz and alkali feldspar, resembling the composition of Na-rich pozzolans. In this framework, the current study focuses on the synthesis of inorganic polymers made of aplite and metakaolin as precursors. In addition, the production of synthetic Na-waterglass is also tested as candidate soluble silica donor in geopolymer systems, through the hydrothermal treatment of aplite. The obtained results confirm that inorganic polymers produced by hydrothermally treated aplite (HTA) and metakaolin (MK) lead to materials with enhanced compressive strength values compared to specimens produced by untreated aplite and MK. At the same time, sodium silicate solution from aplite and commercially available waterglass present similar properties, indicating that aplite can be used as alternative raw material in the production of sodium silicates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within decades of industrial activities, a huge amount of stored industrial wastes in landfills and untapped side-streams have been accumulated in Europe, constituting an enormous wealth that waits to be unlocked and valorized. Extensive research has been done to investigate the possibility of utilizing a variety of industrial solid residues or wastes [1,2,3,4,5]. However, little research has targeted utilization of natural aluminosilicate type rocks as a source material for geopolymerization, a green technology based on an exothermic heterogeneous chemical reaction between a solid aluminosilicate raw material and an alkali metal silicate solution under mild conditions [2, 6,7,8,9,10,11,12]. In this point of view, the aplite rock, that is found worldwide, mainly in Japan, Russia, U.S.A, Norway and Finland [13], could be an alternative natural aluminosilicate source to produce high added value materials using the geopolymerization technology.
Aplite is an intrusive rock in which quartz and alkali feldspar (microcline and albite) are the dominant components. Aplite is usually Na-rich and contains SiO2 and Al2O3 which seem to be promising for being utilized in the development of aplite-based geopolymer materials. However, the high SiO2 aplite content is attributed to crystalline silicious phases with no presence of amorphous silica, indicating limited reactivity of the sample, that prohibits the possibility of further utilization as silicious raw material for geopolymer production. This necessitates the need for further treatment on aplite to liberate reactive Si in the system, promoting the geopolymerization process.
The broad chemistry of raw materials that are used currently in geopolymers, render aplite a promising precursor to be used either as main silicious source (after appropriate treatment to convent the crystalline silica content into amorphous silica) or as secondary raw material in geopolymer production. Recent studies have used aplite as a source material or addition for the synthesis of cementitious [14], ceramics [15] and geopolymers, along with the addition of blast furnace slag and micro silica, activated by potassium and silicate solution-containing systems [16, 17]. However, the addition of an industrial by product, namely a slag, complexes the geopolymer matrices (including potentially heavy metals) preventing the development of a final product with reproducible properties (e.g.,, compressive strength) [18]. Thus, the current study focuses on the selection of aplite as secondary raw material for the development of geopolymer materials with good mechanical properties and decreased environmental impact. At the same time the production of a synthetic sodium silicate solution is also tested after hydrothermal treatment of raw aplite, as an alternative suggestion to the commercial Na-waterglass.
Materials and methods
Laboratory/analytical equipment and reagents
The synthesis of geopolymer pastes was performed using a Hobart mixer under intensive agitation. The fresh pastes were molded in 50 mm cubic molds (3 specimens per mold), sealed with an organic membrane and cured at laboratory furnaces.
The dissolution of aplite in sodium hydroxide solution was studied in a 400–mL Inconel autoclave reactor, connected to a mechanical stirrer with controlled speed. Ceramic Buchner funnels and filter-paper types of MN 640 d and MN 615 ¼ were used for solid/liquid separation. Typical borosilicate glassware was used for volumetric measurements and plastic bottles were used for the storage of the solutions.
Reagent grade sodium hydroxide (Merck Chemicals, Darmstadt, Germany, 99% purity) was used as the alkaline activator prepared by dissolving sodium hydroxide pellets in deionized water. Sodium silicate solution (Merck, extra pure, SiO2: 26.65 wt%, Na2O: 8.35 wt%, d: 1.35 g/cm3) was also added in the liquid phase of the mixture in selected mix designs.
The particle size distribution (PSD) of aplite was determined by laser diffraction using a Malvern Mastersizer Analyzer.
The XRF analysis of the aplite was performed with a SPECTRO XEPOS ED-XRF Analyzer. Quantitative elemental chemical analysis of wet samples (produced by dissolution of aplite) was performed by Atomic Adsorption Spectroscopy (AAS) and Inductive Couple Plasma Optical Emission Spectroscopy (ICP–OES). In more detail, AAS analysis was performed with a PerkinElmer™ PinAAcle 900 T Atomic Adsorption Spectrometer. ICP–OES analysis was performed with a PerkinElmer™ Optima 800 Optical Emission Spectrome-ter.
Crystallographic analysis of aplite was performed in a Malvern-PANalytical™ X’Pert Pro diffractometer, with CuKa radiation (V = 40 kV και I = 30 mA).
Phase identification of aplite was performed with Bruker™ DIFFRAC.EVA software and use of ICDD™ Diffraction databases PDF-4+ και PDF-4 Minerals. Quantitative analysis of the identified phases was performed with the Bruker™ DIFFRAC.TOPAS software.
The compressive strength of produced geopolymers performed by the servo-hydraulic loading machine Advantest 9, after 7 and 28 days of hardening according to ASTM C109 [19]. For compressive strength measurements, three cubic specimens of 50 mm edge were used for each material and the mean value of the three measurements was reported as the final compressive strength of the material. In order to achieve smooth and uniform dimensions appropriate for mechanical performance evaluation, the measurements took place after cutting the final materials using a band saw.
Materials used
Prior to any analytical technique and synthesis of the geopolymers, aplite was ground to a small particle size in a ball mill with porcelain milling media, to avoid any potential contamination and then dried at 105 °C for at least 24 h. The d10, d50, and d90 values, which are intercepts for 10%, 50%, and 90% of the cumulative mass of aplite after milling, are presented in Table 1.
The raw materials used for the synthesis of geopolymer slurries consist either of solely aplite originated from the mining processing of Namsskogan, Norway, or of aplite and metakaolin (Al2O3: 42 wt% and SiO2: 53.9 wt%) produced in the laboratory after thermal treatment of pure kaolinite from VWR (Bole White) at 750 °C. The chemical and mineralogical analysis of aplite is shown in Table 2 and Fig. 1, respectively.
Subsequently, a quantitative (Rietveld) analysis was also performed and the results are shown in Table 3.
According to the chemical analysis (Table 2), aplite is rich in SiO2 being equal to 87 wt%, while Al2O3 is substantially lower reaching a level up to 6 wt%. CaO, K2O and Na2O are the main impurities in aplite. Mineralogical analysis (Fig. 1) of ground aplite confirms that crystalline quartz is the main host mineral of SiO2 while the remaining amount of silica exists inside the feldspars, more specifically in microcline and albite which are the host minerals of Al2O3 in aplite. This is also confirmed by the quantitative analysis of raw aplite (Table 3) revealing that a major part of the material is quartz, followed by secondary phases attributed to albite, and microcline.
Therefore, based on the characterization results, it is concluded that raw aplite rock is not a promising precursor material for alkali activation for two reasons: (a) Silica exists in crystalline quartz which exhibits very limited dissolution in NaOH solutions under atmospheric conditions [20] and: (b) The remaining silica and alumina found in feldspars (microcline and albite) are non–soluble minerals in NaOH solutions, not only at atmospheric conditions but also under elevated temperatures and pressures.
Given the above conditions, grinding of aplite was considered to be a requisite to improve its dissolution ability by increasing substantially its specific surface area and probably deliberating micronized quartz crystals, exhibiting thus superior dissolution capability. The reason why Na–waterglass and metakaolin are used as additives in the mix designs of geopolymer synthesis is the lack of easily dissolved silica and alumina during geopolymerization, as indicated by the physicochemical characterization of aplite (Table 2 and Fig. 1). Metakaolin is an amorphous, easily dissolved material in NaOH solutions, being, therefore, a good precursor for easily dissolved aluminum and silicon in alkali activation [21].
Experimental methodology
Two different routes are followed for the implementation of the experimental work based on the valorization of aplite either as: (a) Raw material/filler to produce geopolymers, or as: (b) A precursor to produce synthetic sodium silicate solution, after hydrothermal treatment of aplite, with direct use in geopolymer production: (a) Aplite as a raw material/filler for geopolymer production: Aplite and/or aplite-metakaolin blend is activated with NaOH and/or sodium silicate solution. Preliminary experiments on using ground aplite powder as the sole precursor for the synthesis of geopolymers are performed according to the following procedure: Ground aplite is mixed with an alkali activator (either 8 M NaOH solution or a doped NaOH solution with Na-waterglass) at a solid to liquid ratio (S/L) in the range 2.5–4.5 g/mL [22]. The selected activator’s concentration is equal to 8 M according to published studies, reporting that at lower alkalinity level, the OH− ion amount is inadequate to facilitate the dissolution of silicate and aluminate species that promote polymerization [23]. On the other hand, under higher alkaline conditions the oligomeric silicate species lose their stability in favor of mononuclear silicate species [6, 24]. The resulting paste is casted in cubic molds (triple samples) and cured at 70 °C under atmospheric pressure for several days (7–10 days). A second experimental series is also performed, where part of aplite is substituted with metakaolin to boost the paste in dissolved silica (e.g.,, 70A–30MK denotes that the solid precursor is composed by 70 wt% aplite and 30 wt% metakaolin) and the solid to liquid ratio is 1.1 g/mL. The curing temperature is increased to 80 °C for 3 days and then the materials are demolded and further cured at ambient temperature (4 or 25 days) for compressive strength measurements. The experimental conditions are summarized in Table 4, where five different mix designs are presented. (b) Hydrothermal treatment of aplite for synthetic waterglass production with direct use in geopolymer synthesis: A new series of experiments is performed using aplite at autoclaved conditions to examine the option of producing synthetic sodium silicate solution that could be directly used (instead of commercial waterglass) for the production of geopolymers. The selected system to study is the one where the wt% substitution of aplite with metakaolin is 50% (50A–50MK). The whole amount of aplite needed for the paste preparation is added in an Inconel autoclave (detailed description at chapter 2.1) at a solid to liquid ratio of 0.5 g/mL with 314 mL of 8 M NaOH solution, heated in the temperature of 200 °C for 2 h at a pressure of 15 bars. After the end of the hydrothermal treatment, a small amount of liquor is withdrawn, filtered and sent for dissolved Si chemical analysis. Then the pulp is mixed thoroughly with the proper amount of metakaolin to have the 50 wt% substitution of aplite, forming a paste with a solid to liquid ratio of 1.1 g/mL (this ratio has been calculated by adding the initial aplite mass that was fed to the autoclave with the remaining mass of aplite and the mass of added metakaolin to form the 50A–50MK solid precursor). The pastes are casted and cured for 3 days at 80 °C and then remain additionally 4–25 days at ambient temperature before the measurement of compressive strength. The experimental conditions are summarized in Table 5.
Results and discussion
Valorization of aplite as raw material/filler for geopolymers production
According to the preliminary experiments performed using aplite as the main raw material to produce geopolymer specimens, the pastes failed to set under any of the studied conditions even if high curing temperature (70 °C) and prolonged curing time (up to a week) were applied. The results indicate and support the lack of minerals dissolution (quartz, microcline and albite) from aplite under the mild activation conditions applied in laboratory. Considering that in a typical geopolymer paste the molar ratio SiO2/Na2O should be in the range of 3.6–5 (w/w) and the molar ratio SiO2/Al2O3 in the range of 3.5–4.5 (w/w) [22], the absence of any significant dissolution, principally of quartz and secondarily of (Na, K, Ca) feldspars in NaOH activator inhibit the setting procedure of the paste. Even in the case of doping the activating solution with Na-waterglass that has a SiO2/Na2O molar ratio 3.3 (w/w), the absence of any significant quartz dissolution keeps the SiO2/Na2O molar ratio lower than the value of 3.5 (w/w) and thus hinders the setting of the paste.
To overcome the setting difficulty observed above, the strategy of doping aplite with metakaolin was adopted in the second series of tests (Table 4), where part of aplite was substituted by metakaolin. In this experimental series, metakaolin works as an active precursor and thus donor of dissolved aluminum and silicon ions to the geopolymer network, while aplite behaves as a filler. Five different mix designs are examined at a substitution ratio of MK–A in the range of 10–50 wt%. The selected alkaline activator is either NaOH or NaOH with sodium silicate solution. The compressive strength values of the produced specimens are presented in Fig. 2.
It is seen from the results that the substitution of aplite with metakaolin to an amount as high as 30 wt% (MD1 & MD2) leads to pastes that cannot set. Higher substitution to 50 wt% (MD3), results in pastes that set within the timeframe of 3 days with compressive strength after 7 and 28 days 2.2–2.6 MPa, respectively. This can be explained by the molar ratios of produced pastes compared to the typical molar ratios encountered in geopolymer pastes, according to the literature [22]. According to calculations (Table 6), the molar ratios of the pastes in MD1 and MD2 are far away from the corresponding typical MR values. The pastes are deficient in silica and alumina as the Na2O/SiO2 (3.71 (w/w) for MD1 and 1.24 (w/w) for MD2) and Na2O/Al2O3 (8.10 (w/w) for MD1 and 2.70 (w/w) for MD2) molar ratios are extremely higher than the corresponding typical values in geopolymer pastes (Na2O/SiO2: 0.2–0.28 (w/w) and Na2O/Al2O3: 0.8–1.2 (w/w)). The molar ratios in the paste from MD3 that set are closer to the typical values although are still far from them (Na2O/SiO2: 0.74 (w/w) and Na2O/Al2O3: 1.62 (w/w)). Metakaolin is not enough to offer to the paste soluble silica and alumina. It helps the system to start polymerization and give solidified compact materials with low compressive strength but the SiO2/Al2O3 molar ratio of 2.18 (w/w) will be always lower than the typical value of 3.5–4.5 (w/w) (almost half the typical value) indicating that the paste must be doped with higher amount of soluble silica.
If the system is dopped with soluble silica by using Na-waterglass (MD4), the molar ratios will be within the typical values and the compressive strength of material will almost quadruple (Fig. 2) reaching a value of 8.7 MPa at 7 days and 9 MPa after 28 days. The molar ratios SiO2/Al2O3: 4.34 (w/w) and Na2O/Al2O3:1.16 (w/w) for MD4 are very close to the highest limit of the typical molar ratio values (SiO2/Al2O3: 3.5–4.5 (w/w) and Na2O/Al2O3: 0.8–1.2 (w/w)) indicating that doping with soluble alumina is necessary to improve further the properties of geopolymer materials. To test this observation, a new experiment with 30 wt% aplite and 70 wt% metakaolin in the presence of Na-waterglass in the activator (MD5) was performed. Alumina doping took place through the increased amount of metakaolin in the mixture of solid precursors. The molar ratios SiO2/Al2O3: 3.59 (w/w) and Na2O/Al2O3: 0.85 (w/w) in this test are located closer to the lower limits of the typical molar ratio values indicating a substantial doping with soluble alumina. Under those conditions the mechanical strength of produced material improved substantially reaching a value of 27 MPa at 7 days followed by 30 MPa after 28 days, revealing the substantial role that soluble aluminum plays in the geopolymer network of material.
The produced materials coming out by MD3, MD4 and MD5 experiments (with successful setting) are depicted in Fig. 3.
It is evident that doping of aplite with metakaolin as well as Na-waterglass, results in well-shaped and structured materials with appreciable mechanical properties (Fig. 2). This type of doping is not the most cost-effective option (metakaolin price: 300–400 $/t, Na–waterglass: 700–900 $/t in solid state) and thus, alternative solutions must be found to render the aplite-based geopolymers less costly and thus more attractive.
Hydrothermal treatment of aplite for synthetic waterglass production with direct use in geopolymers
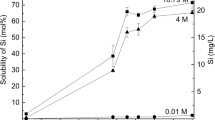
To decrease or even eliminate the doping with Na-waterglass, silicon must be transferred to the paste by the aplite dissolution in NaOH solutions. To achieve this, the hydrothermal treatment of aplite in autoclave is necessary as it has been confirmed by literature [25]. At 200 °C and under the given conditions (Table 5) the hydrothermal treatment of aplite is performed, resulting in a viscous aqueous phase with dissolved silicon content of 146 g/L. By assuming that the density of the liquor after the hydrothermal treatment has a value of 1.35 g/mL (which is a common density for a Na-waterglass solution), then the silica content of the aplite waterglass reaches the value of 23.2 wt%. In any case, this measurement indicates that the hydrothermal treatment of ground aplite can offer to the system appreciable amount of dissolved silica, forming a type of synthetic waterglass that has the potential to completely substitute the commercial one. The compressive strength of 13 MPa achieved after 7 days followed by 15 MPa after 28 days, is directly comparable and higher with the one of 8.7 MPa achieved with the addition of Na-waterglass (MD4 in Table 4) and again dictates that hydrothermally treated aplite has the potential to act as a substitute of Na-waterglass. The experiment MD6 (50HTA–50MK) in Table 5 and MD4 (50A–50MK + WG) in Table 4 have similar molar ratios and for this reason give enhanced values of compressive strengths. The physical appearance of the material MD6: 50HTA–50MK is shown in Fig. 4. The material is compact and very well shaped and structured.
The comparison of 28 days compressive strength of MD6: 50HTA–50MK (15 MPa) with the respective mix formulation of 50A–50MK (2.6 MPa) shows that the hydrothermal treatment of ground aplite improves the strength of material at almost 6 times at the expense of the cost of the hydrothermal treatment in autoclave. In the formulation 50HTA–50MK the value 1.62 (w/w) of the molar ratio Na2O/Al2O3 indicates that the system is deficient in Al2O3 (as this molar ratio Na2O/Al2O3 value in typical geopolymers in in the range of 0.8–1.2 (w/w)) and thus must be doped with easily dissolved Al2O3 to improve the geopolymerization performance. To make such an adjustment in the composition of the paste is a difficult issue, as a good donor of Al2O3 must be sought that its addition to this system wouldn’t affect substantially the Na2O and SiO2 content. This type of modification was not investigated within the frame of this work, but it is something that could be done as a future step in the aplite hydrothermal treatment optimization. On the other hand, the hydrothermal treatment of ground aplite leads to a synthetic aplite Na-waterglass as indicated by the measured Si content, reaching the value of 146 g/L (~ 70% Si extraction based on raw aplite chemical analysis). The pH of sodium water glass that was used for the synthesis of the fresh mortars is equal to 11.8. The sodium silicate solution that was produced after the hydrothermal treatment of aplite reaches a pH value equal to 12.3. Thus, the treated aplite leads to a synthetic waterglass, possessing almost similar properties with the commercial one, as confirmed by Table 7.
Conclusions
In the current work, aplite was tested as raw material for the production of geopolymers and as a candidate soluble silica donor in geopolymer systems after hydrothermal treatment. Using solely ground aplite as a solid precursor and NaOH solution as alkaline activator, results in unsuccessful setting of pastes and this approach has no potential for geopolymer production. Using a 50/50 mixture of ground aplite with metakaolin as a solid precursor and NaOH solution as alkaline activator (MD3), results in successful setting of pastes and in production of geopolymers with compressive strength of 2.2–2.6 MPa at 7 days and 28 days, respectively. Using a 50/50 mixture of ground aplite with metakaolin as a solid precursor and NaOH solution doped with Na-waterglass (as soluble silica and alumina donor) as alkaline activator (MD4), results in successful setting of pastes and in production of geopolymers with improved compressive strength of 8.7–9 MPa at 7 days and 28 days, respectively. By applying higher aplite substitution with metakaolin and doping with waterglass (MD5), an excellent material was produced that had 27 MPa compressive strength at 7 days and 30 MPa at 28 days. This test elucidates the need for doping aplite with enough soluble aluminum and silica. The doping with silica can also be achieved directly by performing hydrothermal treatment of aplite at elevated temperatures to produce a synthetic Na-waterglass. The doping with aluminum needs a proper donor, probably fine alumina trihydrate.
Using a 50/50 mixture of hydrothermally treated ground aplite with metakaolin as a solid precursor and NaOH solution as alkaline activator (MD6), results in successful setting of pastes and in production of geopolymers with improved compressive strength of 13 MPa at 7 days and 15 MPa at 28 days (compared to the same recipe MD4 where Na-waterglass was added as soluble silica donor with compressive strength values of 8.7–9 MPa). The hydrothermal treatment of ground aplite (200 °C, 2 h, 8 M NaOH) in autoclave, leads to a synthetic Na-waterglass with characteristics very similar to the commercial Na-waterglass. As a conclusion, the most promising formulation is the 50HTA–50MK in NaOH activator that gave 13–15 MPa compressive strength at 7–28 days. This formulation needs optimization toward: (a) the improvement of the paste composition (e.g., by doping with dissolved alumina) that would potentially increase the mechanical strength and (b) the decrease in the cost of materials (e.g., by optimization of the hydrothermal treatment of aplite, substitution of metakaolin partially or totally with silica fumes, and/or kaolin).
References
Panias D, Giannopoulou I, Perraki T (2007) Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surf A 301:246–254. https://doi.org/10.1016/j.colsurfa.2006.12.064
Pontikes Y, Machiels L, Onisei S, Pandelaers L, Geysen D, Jones PT, Blanpain B (2013) Slags with a high Al and Fe content as precursors for inorganic polymers. Appl Clay Sci 73:93–102. https://doi.org/10.1016/j.clay.2012.09.020
Komnitsas K, Zaharaki D, Perdikatsis V (2009) Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J Hazard Mater 161:760–768. https://doi.org/10.1016/j.jhazmat.2008.04.055
Panias D, Balomenos E, Sakkas K (2016) The fire resistance of alkali‐activated cement‐based concrete binders. In: Pacheco‐Torgal F, Labrincha J, Leonelli C, Palomo A, Chindaprasit P (Ed) Chapter 16 in book handbook of alkali‐activated cements, mortars andconcretes, Woodhead Publishing 2016, pp 423‐463
Tsaousi GM, Douni I, Panias D (2018) experimental evaluation of efficient Si dissolution from perlite at low level activator’s concentration. Minerals 8(4):160. https://doi.org/10.3390/min8040160
Barbosa VFF, MacKenzie KJD, Thaumatutgo C (2000) Synthesis and characterization of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers. Int J Inorg Mater 4:309–317. https://doi.org/10.1016/S1466-6049(00)00041-6
Xu H, Van Deventer JSJ (2000) The geopolymerization of alumino-silicate minerals. Int J Miner Process 3:247–266. https://doi.org/10.1016/S0301-7516(99)00074-5
Palomo A, Grutzeck MW, Blanco MT (1999) Alkali activated fly ashes-a cement for the future. Cem Concr Res 8:1323–1329. https://doi.org/10.1016/S0008-8846(98)00243-
Davidovits J (1994) Properties of geopolymer cements. In: Proceedings of the first international conference on alkaline cements and concretes, geopolymer institute, Kiev, Ukraine, pp 131–149
Cheng TW, Chiu JP (2003) Fire resistant geopolymer produced by granulated blast furnace slag. Miner Eng 3:205–210. https://doi.org/10.1016/S0892-6875(03)00008-6
Cundi W, Hirano Y, Terai T, Vallepu R, Mikuni A, Ikeda K (2005) Preparation of geopolymeric monoliths from red mud-PFBC ash fillers at ambient temperature. In: Proceedings of the world congress geopolymer, Saint Quentin, France. pp 85–87
Tsaousi GM, Panias D (2021) Properties and performance of slag-based, geopolymer foams. Minerals 11:732. https://doi.org/10.3390/min11070732
Bishop AC (1989) Aplite. Petrology encyclopedia of earth science. Springer, Boston. https://doi.org/10.1007/0-387-30845-8_14
Gholipour S et al (2017) The effect of aplite on the mechanical properties of cement mortar». Constr Build Mater 155:390–397
Palchik V (2015) Aplite as a source of raw materials for the production of glass and ceramics». Glass Ceram 72(5–6):214–217
Khalifeh M, Saasen A, Vrålstad T, Larsen HB, Hodne H (2015) Experimental study on the synthesis and characterization of aplite rock-based geopolymers. J Sustain Cem Based Mater. https://doi.org/10.1080/21650373.2015.1044049
Samindi S, Samarakoon S, Kakay S, Soli J, Knutsen M, Emil J, Knutsson, Tobias (2016) Performance evaluation of aplite rock based geo-polymer binder. In: Proceedings of international RILEM conference on materials, systems and structures in civil engineering conference segment on concrete with supplementary cementitious material, Lyngby, Denmark
Tsaousi GM, Sakkas KM, Panias D (2022) Development of advanced materials from industrial waste, with high thermal performance. Constr Build Mater 315:125779. https://doi.org/10.1016/j.conbuildmat.2021.125779
Designation: C 109/C 109M–02, Standard test method for compressive strength of hydraulic cement mortars (Using 2-in. or [50-mm] cube specimens), ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428–2959, United States
Thornton SD (1988) Dissolution and condensation of silica in alkaline solution. SPE Res Eng 3:743–752. https://doi.org/10.2118/13601-PA
Rahier H, Wastiels J, Biesemans M, Willlem R, Van AG, Mele BV (2007) Reaction mechanism, kinetics and high temperature transformations of geopolymers. J Mater Sci 42:2982–2996. https://doi.org/10.1007/s10853-006-0568-8
Davidovits J (1991) GEOPOLYMERS: inorganic polymeric new materials. J Therm Anal 37(8):1633–1656. https://doi.org/10.1007/BF01912193
Komnitsas K, Zaharaki D (2007) Geopolymerisation: a review and prospects for the minerals industry. Miner Eng 20(14):1261–1277. https://doi.org/10.1016/j.mineng.2007.07.011
Singh PS, Bastow T, Trigg M (2005) Structural studies of geopolymers by 29Si and 27Al MAS-NMR. J Mater Sci 40(15):3951–3961. https://doi.org/10.1007/s10853-005-1915-x
Pfeiffer T, Sander SAH, Enke D, Roggendorf H (2019) Hydrothermal dissolution of low-quartz in sodium hydroxide lyes: kinetics and equilibrium. Chem-Ing-Tech 91(1):92–101. https://doi.org/10.1002/cite.201800092
Acknowledgements
The authors acknowledge support from Aplitt AS company in Norway.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Georgia Maria Tsaousi and Georgia Flesoura designed and performed the experiments under the supervision of Professor Dimitrios Panias; Georgia Maria Tsaousi analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Andréa de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsaousi, GM., Flesoura, G. & Panias, D. Valorization of aplite in alkali-activated materials. J Mater Sci 59, 8160–8168 (2024). https://doi.org/10.1007/s10853-024-09668-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09668-4