Abstract
Pd and TiO2 supported on functionalized multiwall carbon nanotubes (f-MWCNTs) catalysts were investigated in formic acid electrooxidation reaction in direct formic acid fuel cell. TiO2 (5–60 wt.% loading) on f-MWCNTs was deposited using microwave-assisted hydrothermal method. 20 wt.% of Pd was deposited on TiO2/f-MWCNTs by reduction of palladium (II) chloride salt with sodium borohydride. Catalysts’ structure and composition were characterized by XRD, STEM, HR-TEM, TGA, XPS/XAES (Pd, Ti, O spectra features, density of valence states, Auger parameters). Average crystallite size of Pd and TiO2 from XRD (3–4 nm) agrees with those by HR-TEM (3–5 nm). Low TiO2 coverages (below 32wt.%) show smaller crystallites due to increased surface hydrophilicity, higher amount of TiO2 oxygen vacancies with attached Pd nanoparticles, increased density of valence states of strong Pd–TiO2 interface. In contrary, the higher coverages indicate lower amount of Pd–O–Ti, Ti–O–C, Pd–O–C interfaces, with electron charge transfer from TiO2 to f-MWCNTs, and to Pd. Catalysts activity (40–106 mWmgPd−1) and stability (5–240 h) are enhanced at low TiO2 coverages (4–8 wt.%) due to a strong Pd-TiO2 interface on oxygen vacancies, improved electron transport and a high active surface area. Oscillatory self-cleaning mechanism of Pd is due to oxidation by -OH groups (TiO2, f-MWCNTs), and hydrogen and CO spillover.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, the low-temperature fuel cells have attracted a lot of attention due to their use as clean energy sources in portable devices, vehicles and stationary power supplies. Among the low-temperature fuel cells the direct formic acid fuel cell (DFAFC) displays several advantages over traditional hydrogen and methanol fuel cells, including higher energy density, better oxidation kinetics, greater energy conversion efficiency, lower toxicity and fuel crossover [1,2,3,4,5]. The dual-path mechanism of formic acid electrooxidation reaction (FAOR) presents two distinctive pathways, i.e., a direct and indirect. In the direct pathway dehydrogenation process leads to formation of a reactive intermediate (COOH*), which is oxidized to CO2 (Eq. 1):
The indirect pathway leads to dehydration process, where CO species (COads) are adsorbed at catalyst surface being at higher potentials oxidized to CO2 by adsorbed oxygenated species (Eq. 2):
In contrary to the predominant dehydration indirect pathway on Pt the FAOR on Pd atoms at lower potential [6] proceeds mainly via the direct pathway through dehydrogenation reaction.
However, palladium catalysts quickly lose their activity and stability, especially in the presence of even a small content of impurities in formic acid, what leads to indirect pathway, where catalyst is deactivated via poisoning the surface by the reaction intermediates (e.g., CO), C dissolved in Pd, or by other adsorbed species from contaminations (e.g., OHad, ClO4, ad and Clad) [7, 8].
Literature reports several strategies employed to enhance the direct pathway of the reaction for avoiding the catalyst surface poisoning and increasing the efficiency and durability of Pd catalyst in a FAOR. To design the catalytic properties dependent on various factors such as surface area and porosity, nanoparticle crystallinity, shape, size, dispersion, exposed facets, electronic structure, chemical surrounding and electron conductivity different synthesis routes were proposed promoting: (i) the electronic properties to weaken the Pd-COads bond, (ii) strengthening the metal–support interaction to improve the electron transport, (iii) oxidation of the adsorbed COads from the Pd surface and (iii) surface functionalization to obtain specific chemical and structural properties of a catalyst such as nanoparticle size and dispersion. Pd catalyst systems have been designed by modification with transition metals [3, 9,10,11,12], transition metal oxides [13,14,15,16,17,18,19,20,21], various carbon supports [8, 9, 13, 14, 16, 21,22,23,24,25,26,27] and applying synthesis routes to tailor various chemical and structural surface properties of the catalysts (doping, NPs crystallinity, size, shape, dispersion, etc.) [24,25,26].
Rice et al. [28] attributed production of higher current densities by Pd–Pt catalyst operating in DFAFC to promoting direct pathway dehydrogenation reaction. Addition of Au, Pb or Bi to Pd catalysts revealed a higher activity and stability in FAOR than in commercial Pd catalysts [9,10,11,12] explained by the direct pathway promoted by the electronic and third-body effect [12]. Pd and Pt nanoparticles (NPs) supported on metal oxides exhibited enhanced electrocatalytic performance, what was reported for the first time by Tauster et al. [18] and attributed to the strong metal–support interaction (SMSI) effect. According to the Brewer theory [29], titanium dioxide has the hypo-d-electron character, which interacts with the inert noble metals like Pd changing additionally its catalytic activity. This modification of catalytic activity has been explained by considering Hammer and Norskov’s concept [30], in which metal reactivity changes through modification of adsorbate surface interaction energy due to a shift of the local d-band position relative to the Fermi level. The presence of dopants such as B or N atoms strengthening the interaction between the catalyst and support resulting in a better electron transfer was reported recently [26, 27], similarly to the presence of transition metal oxides, –OH groups, e.g., from TiO2 [19,20,21], facilitating oxidation of COads from Pd due to spillover of hydrogen [15] and CO [21].
Recent literature on modified Pd-based catalysts on different supports in FAOR reports enhanced activity and stability in comparison with Pd on carbon black commercial catalyst [19,20,21,22,23,24,25,26,27]. The supports proposed, and additional functionalization during preparation, play a role in: (i) modification of electronic properties of the catalyst, (ii) increase of surface area and porosity, (iii) increase of metal to metal oxide ratio, (iv) delivery of oxygen and hydrogen for intermediate oxidation/reduction reactions and (v) preparation of catalysts NPs of the least size, highest dispersion, selective size and exposed facets. Among various carbon nanomaterials Pd and/or Pd-transition metal oxides catalysts were prepared on supports such as carbon multiwall nanotubes (MWCNTs), carbon nanofibers (CNFs), reduced graphene oxide (rGO), graphitic carbon nitride (g-C3N4) [19,20,21,22,23,24,25,26]. For Pd and/or PdCo NPs (size of 1.5–5.4 nm) on carbon nanomaterials the mass activity from 1610 mA mgPd−1 to 4833.12 mA mgPd−1 and enhanced stability were reported [24, 25]. Among PdCo NPs (size of 3.65–4.62 nm) on MWCNTs and N-rGO with mass activity of 4833–1010 mA mgPd−1 the Pd1Co1/N-rGO showed the highest activity and stability attributed to the bifunctional effect of Co and SMSI [25]. After 5000 s this catalysts retained 15% of its initial mass activity [25]. PdCo alloy nanocluster on MWCTs catalysts (Pd3Co1/MWCNTs, Pd1Co1/MWCNTs, Pd1Co3/MWCNTs) prepared in deep eutectic solvents of NPs size of 4.3, 4.9, 5.3 showed mass activity toward FAOR of 2410.1 mA mgPd−1, 1752.3 mA mgPd−1, 1255.1 mA mgPd−1, respectively, in comparison with Pd/MWCNTs (7.5 nm, 1262.8 mA mgPd−1) and commercial Pd3Co1/C and Pd/C catalysts (1919.2 mA mgPd−1 and 819.2 mA mgPd−1). Pd3Co1/MWCNTs catalyst exhibited the best anti-CO toxicity at 0.36 V and a long performance with 8% of initial mass activity after 5000 s, what was attributed to lowering of Pd d-band, formation of new active sites and improving the electron transport [31]. Pd/ZrO2/MWCNTs catalysts (8 nm Pd NPs, 4.4 ZrO2 NPs) showed a high durability with specific power decreasing to about 25% of its initial value via spontaneous oscillations of the fuel cell potential attributed to the self-cleaning mechanism of poisoned Pd due to Pd-Zr interface [17]. Pd catalysts (nanoparticle size of 4–6 nm) on amorphous TiO2 coating (layer thickness 2–4 nm) on MWCNTs prepared by sonochemical reaction and applied for FAOR were reported by Shi et al. [19]. The best catalyst characterized by a continuous conformal TiO2 coating with the thickness of ~ 1.5 nm without any exposed MWCNTs surface, showed the mass activity of 2600 mA mgPd−1. The presence of TiO2 coating leads to 5.2 times higher mass activity and 2.2 times higher specific activity than those for Pd/MWCNTs, whereas the peak current density retained about 31% of the initial activity after 5000 s. The enhanced FAOR activity was attributed to SMSI effect resulting from the interface between Pd NPs and TiO2 coating, and the bifunctional mechanism of TiO2. As reported previously, efficiency of Pd-anatase-TiO2 Vulcan XC-72R carbon black composite of Pd nanoparticles (size of 3.5 nm and high dispersion) synthesized by an impregnation-reduction method and investigated toward FAOR, depends on the ratio of Pd to TiO2 [20]. The increased activity was obtained for the ratio of Pd to TiO2 1:1. FAOR on TiO2(001)@Pd(111) catalyst (ultrathin palladium layer exposed to Pd{111} facet with an average thickness of 0.9 nm grown on {001}-faceted anatase TiO2 nanosheets) showed the higher activity, stability and CO tolerance in comparison with Pd/TiO2(001) and a commercial Pd/C catalysts with similar palladium loadings, retaining 15% of its initial activity after 3600 s [21]. This provides a strategy to synthesize highly stable and active supported ultrathin metal catalysts with exposed specific facets [21]. Among other various carbon materials used as a support the polyvinylpyrrolidone (PVP)-capped Pd nanoparticles on the MWCNTs-supported catalysts were shown to be effective in a formic acid dehydrogenation reaction for producing hydrogen [27]. The presence of PVP played a positive role by increasing the hydrophilicity of the materials and NPs size (2.5–3.3 nm), dispersity and enhancing the interface contact between the reactant molecules and the catalytic active sites. Pretreatment of the carrier for supported catalysts can effectively improve SMSI and increase the dispersion of metal [32]. Tuning of TiO2 defects induced by pretreatment in H2/N2, N2, O2/N2 at the high temperature showed that the surface defects anchoring Pd NPs enhance their dispersion [32]. Reduction of TiO2 leads to formation of Ti3+ species inducing SMSI effect, Pd nanoparticles stabilization and efficiency of formic acid oxidation reaction. Nanoflower-like TiO2-supported Pd catalyst of a high concentration of oxygen vacancies and surface hydroxyl groups in comparison with a commercial anatase support Pd catalyst showed improved dispersion and stabilization of Pd species and remarkable efficiency in catalyzing the low-temperature CO oxidation [33]. In FAOR the electron-rich Pd nanoparticles on TiO2 support with TiO2 oxygen vacancy showed 1.41 and 2.72 times larger mass activity than Pd/TiO2 catalyst without oxygen vacancies and a commercial Pd/C, respectively [34]. The most efficient catalyst showed 50% of its initial activity after 5000 s. Experiment and a theory confirm that the oxygen-vacant TiO2 downshifts the d-band center of Pd and facilitates hydrogen spillover accelerating Had desorption from Pd avoiding adsorption of hydrogen species, which serve as the primary intermediators at the active sites of catalyst.
Since TiO2 was shown to enhance FAOR on Pd the fast and easy method for preparation of uniformly dispersed TiO2 nanoparticles on the multiwall carbon nanotubes (f-MWCNTs) surface by microwave-assisted hydrothermal method and Pd nanoparticles by reduction of palladium (II) chloride salt with sodium borohydride was proposed. This method was supposed to lead to preparation of Pd-TiO2/f-MWCNTs catalyst of a very small TiO2 and Pd crystallites and a high surface area for an improved catalytic performance. The influence of TiO2 content on Pd-TiO2/MWCNTs catalyst structural, chemical properties, activity and stability in the DFAFC was investigated. To the best of our knowledge, the influence of TiO2 coverage in the proposed Pd NPs TiO2-coated f-MWCNTs and the catalytic application in the DFAFC have not been yet reported.
Experimental
Samples and preparation
The "as-received" MWCNTs (CNT CO., Ltd., Korea) of average diameter size of 20 nm to 40 nm were purified and oxidized in a boiling concentrated (65%) HNO3 under reflux for 50 h. Then, rinsed with: (i) water until pH of the filtrate was stabilized to pH = 7, (ii) 25% aqueous solution of ammonia and (iii) again water until the pH of the filtrate was stabilized to 7. Further, the sample was dried at room temperature (RT) and then the f-MWCNTs were obtained. The surface area measured using the Brunauer–Emmett–Teller adsorption isotherm (BET) method in the case of the "as-received" and the f-MWCNTs was 177 m2 g−1 and 254 m2 g−1, respectively [35].
The TiO2 nanoparticles on the surface of f-MWCNTs of different concentration (5 to 60 wt. %) were prepared using a microwave-assisted hydrothermal method. Different TiO2 loading on f-MWCNTs was obtained by using an appropriate amount of 1 wt.% titanium tetraisopropoxide (98 + % ACROS Organics) in isopropyl alcohol solution added to the suspension of 0.5 wt.% f-MWCNTs in isopropyl alcohol. Then, the mixture was placed in a Teflon container and heated in the microwave reactor at a pressure of 55 bar, to a temperature of 200 °C for 20 min. The sample was centrifuged, washed by distilled water and dried at room temperature overnight. These samples were denoted as: (i) 5%TiO2, (ii) 10%TiO2, (iii) 20%TiO2, (iv) 40%TiO2 and (v) 60%TiO2, where percent denotes wt.%. Palladium nanoparticles on the surface of TiO2/f-MWCNTs composites were prepared through reduction of PdCl2 (5 wt. % in 10 wt. % HCl, Sigma-Aldrich) with sodium borohydride—NaBH4 (≥ 96% p.a., Sigma-Aldrich). TiO2/f-MWCNTs were dispersed in water to which an adequate amount of aqueous solution of PdCl2 was added. The pH value was adjusted to 10 using NaOH aqueous solution. Next, 1 M NaBH4 solution was added to the mixture and was kept stirring for 1 h. After that, the sample was filtered, washed by distilled water and dried at room temperature overnight. Depending on the content of TiO2, 20 wt.% Pd-TiO2 on f-MWCNTs catalyst composites resulted in composition of: (i) Pd-4%TiO2, (ii) Pd-8%TiO2, (iii) Pd-16%TiO2, (iv) Pd-32%TiO2 and (v) Pd-48%TiO2, where percent denotes wt.%.
Apparatus
The X-ray powder diffraction (XRD) data were collected with hybrid Rigaku Denku (Geigerflex) diffractometer in a Bragg–Brentano configuration using CuKα Ni-filtered radiation, 40 kV and 40 mA tube settings and with stability of 0.01%/8 h. Measurements of each sample were taken in the scattering 2Θ range of 20° to 100° with a step of 0.02° and counting time of 8 s/step.
The scanning transmission electron microscopy (STEM) measurements were taken in a high-resolution scanning electron microscope HITACHI (Hitachi Ltd., Japan) s5500 in scanning transmission mode at 30 keV. High-resolution transmission electron microscopy (HR-TEM) measurements were taken using a Tecnai F20 electron microscope (Thermo Fisher Scientific, Netherlands) at accelerating voltage for 200 keV with 100-µm objective aperture.
The measurements of the X-ray photoelectron spectroscopy (XPS) survey and detailed spectra were taken using the homemade ESA-31 electron spectrometer [36] equipped with a high-energy resolution analyzer, an electron gun of LEG62 (VG Microtech, UK), homemade X-ray sources with the radiation of Al Kα (1486.67 eV) and Cu Kα (8047.82 eV) and Ar ion gun (VG Scientific UK). The spectrometer operates using the fixed retardation ratio (FRR) working mode (retardation ratio for survey spectra was 4 and for detailed spectra that was 16) and the angles of X-ray incidence and photoelectron emission 70º and 0º, with respect to the surface normal of the sample. The catalyst samples were investigated without Ar ion sputtering. For the energy scale calibration of the 10–6 stability (ripple, heat stability, etc.) XPS homemade measurement power supplies, the so-called relative energy calibration method was used, namely instead of absolute calibration we used atomic standards of high purity or spectral purity Cu, Ag and Au metal sheets cleaned by “in situ” Ar ion sputtering. The binding energy (BE) values originating from NPL (UK) [37] and NIST (USA) [38] were accepted as very precisely measured values of Cu 2p, Cu 3p, Ag 3d and Au 4f with absolute calibration in a traceable way with voltage standards. The digital to analog converter (DAC) linearity was very excellent, and the zero offset of the DAC was very stable as well, especially with the recently changed DAC. Offset energy, including spectrometer work function changes, is around 50 meV precision, and the slope linearity is changing in the 10–6 range. In the case of the catalyst samples XPS measurements several eV charging effects were experienced what were corrected assuming linear charging and using reliable and precise values of the C 1 s line for the different carbon bonding states of sp2 and sp3, etc.
The TGA measurements were taken using Mettler Toledo TGA/DSC3 + in the 70 µl alumina crucibles with 30 ml/min air flow and 10 °C/min heating rate in the range of 20 to 1000ºC.
Fuel cell tests
The electrode assemblies were fabricated using the "direct painting" technique to apply a catalyst layer on a carbon cloth diffusion layer with an area of 5 cm2. The anode and cathode catalyst inks were prepared by dispersing the catalyst in water, where a 5 wt. % solution of Nafion in light alcohols (Du Pont) was added. The electrodes were obtained by painting the inks on carbon cloths and hot-pressing (0.8 kg cm−2, 130° C, 30 min). In the assembly of the fuel cell the Nafion 115 membrane was placed between the cathode and the anode (Fig. S1). For the preparation of the anodes untreated carbon cloth (Carbon Cloth CC6 Plain, Fuel Cell Earth) was used, applying appropriate Pd content to obtain loading of 0.5 mg cm−2. The commercial catalyst 60 wt. % Pt/Vulcan (Premetek Co.) on the cathode side was applied. The ink was painted on the wet-proofed Teflon™-coated carbon cloth (Carbon Cloth CC4 Wet Proofed, Fuel Cell Earth), with Pt loading of 4 mg cm−2. The anode catalysts (Pd-TiO2/f-MWCNTs) performance was investigated from the measurements of galvanostatic polarization curves for formic acid–air cell with current of 40 mA per 1 mg of Pd. The cell was operating at room temperature and ambient pressure with 3 M HCOOH (85% HCOOH pure for analysis, AVANTOR Performance Materials) fed to the anode at a flow rate of 0.5 ml min−1, and the air was delivered to the cathode with a flow rate of 800 cm3 min−1. The initial activity and the stability of the catalysts were tested on the anode of the homemade DFAFC [39, 40]. The activity or maximum power density per mass of Pd catalysts, PmgPd, is determined in galvanostatic measurements, where an alternating current or current density per mass of Pd catalyst (ImgPd) is applied and voltage (V) measured. Maximum power density, i.e., PmgPd = I mgPd x V, determines the specific power density of DFAFC. Changes of maximum power density with time determine the catalyst stability.
Electrochemical measurements
Catalyst ink preparation
Electrochemical measurements were taken for 5 catalysts: Pd-4%TiO2, Pd-8%TiO2, Pd-16%TiO2, Pd-32%TiO2 and Pd/f-MWCNTs as a reference catalyst. For all the samples the ink was prepared by obtaining a suspension of the catalyst (5 mg ml−1) in a mixture of water, isopropanol (1:1 volume) and Nafion solution (5% in lightweight alcohols). Generally, for 2.5 mg of catalyst 245 μl of water and isopropanol and 10 μl of Nafion were added and sonicated for 15 min to obtain a well-dispersed suspension. Next, 5 μl of the ink was deposited on a polished glassy carbon electrode (3 mm diameter). After drying at RT, the electrodes were used in electrochemical measurements.
Electrochemical measurements
Cyclic voltammetry measurements were taken in a three-electrode setup (using Biologic SP-200 potentiostat), where glassy carbon with deposited catalyst was used as the working electrode, platinum wire as the counter electrode and Ag/AgCl as the reference electrode. First, the measurements were taken in a deaerated (purged with argon for 15 min) 0.1 M HClO4 electrolyte in a potential range -0.2 – 0.8 V vs. Ag/AgCl with a 10 mV/s scan rate. Next, for all the prepared electrodes the measurements were taken in a deaerated (purged with argon for 15 min) 0.1 M HClO4 + 3 M HCOOH electrolyte in a potential range -0.2 – 0.8 V vs. Ag/AgCl with increasing scan rates (10 mV/s, 20 mV/s, 50 mV/s, 100 mV/s, 200 mV/s).
Temperature-dependent measurements
Temperature-dependent measurements were taken using the Baltic FuelCells® quick connect fixture qCF FC5 with BioLogic potentiostat SP-150e and 5A booster VMP3B-5. Anode loading was 0.5 mg cm−2 of Pd with 3 M HCOOH fed to the anode at flow rate of 3 ml min−1. Cathode loading was 4 mg cm−2 of Pt at air flow of 800 cm3 min−1. Linear potential sweeps of the DFAFC proceeded with a scan rate of 10 mV s−1 at temperatures of 30 °C, 40 °C, 50 °C, 60 °C and 80 °C. The method for membrane and electrode assembly (MEA) preparation was discussed in the fuel cell tests section.
Results and discussion
Composition and structural properties by STEM, HR-TEM and XRD
The amount of TiO2 and Pd was confirmed by thermogravimetric analysis (TGA) from the values of weight loss (Fig S2a, b). Desorption of adsorbed water and decomposition of carbon–oxygen groups of f-MWCNTs proceed in the temperature range of 100–700 °C [41]. TiO2 decomposes in the range of 180–420 °C [42], whereas Pd oxides decomposition is above 900 °C [43]. Ti (due to decomposed TiO2) content is determined from the difference of weight loss between TiO2/f-MWCNTs and f-MWCNT above 700 °C (Fig. S2a), whereas Pd content is evaluated from the difference between weight loss for Pd/TiO2/f-MWCNTs and f-MWCNT above 900 °C (Fig. S2b). TGA results confirm the composition assumed in chemical preparation procedure.
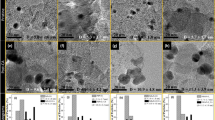
The STEM images of 20 wt.% Pd-8 wt.% TiO2/f-MWCNTs indicate heterogeneously distributed nanoparticles of agglomerated TiO2 and Pd on the f-MWCNTs surface (Fig. 1a). It is not possible to distinguish TiO2 and Pd due to the limited resolution of the STEM microscope. High-resolution TEM (HR-TEM) micrographs show light gray and darker images of nanoparticles, which can be attributed to TiO2 and Pd nanoparticles, respectively. The TiO2 nanoparticles and their agglomerates of size about 5–10 nm are shown on the f-MWCNTs, whereas Pd nanoparticles of the size about 3–5 nm are deposited on f-MWCNTs and TiO2 nanoparticles (Fig. 1b, c).
The XRD patterns recorded for Pd-TiO2/f-MWCNTs catalysts (Fig. 2) show peaks of: (i) the f-MWCNTs, (ii) titanium dioxide anatase phase (JCPDS 12–2172) and (iii) fcc palladium phase (JCPDS 41–1043). The reflexes from Pd and TiO2 were used for determining the average crystallites size (Table S1).
The average crystallite size determined from XRD for Pd and TiO2 is in the range of 3–4 nm and increases slightly with titanium dioxide concentration above 32 wt.% (Table S1). The Pd crystallite size estimated from XRD (3–4 nm) is reasonably consistent with that determined from HR-TEM measurement, i.e., 3–5 nm (Fig. 1b, c). The slightly larger size shown by HR-TEM may be due to Pd oxides overlayer on Pd crystallites. Much larger size of TiO2 nanoparticles reported from HR-TEM (5–10 nm) may result from the presence of amorphous TiO2 phase and/or crystallites agglomeration.
Surface coverage and nanoparticle size by XPS
The analysis of TiO2 and Pd nanoparticles surface coverage on f-MWCNTs and their size was carried out using XPS-QUASES-Analyze software [44] applied for the experimental spectra of Ti 2p3/2–1/2 and Pd 3d5/2–3/2 including inelastic background in the range of 970–1060 eV and 1050–1160 eV (Fig. 5, S3). No BE value calibration is applied in this approach. The values of surface coverage and nanoparticle size resulting from the fitting of the experimental spectra to the spectra of TiO2 and Pd standards (Fig. 3, Fig. S3) were obtained applying the active substrate model and the inelastic mean free path values (IMFP) from the TPP-2 M formula [45] (Table S2).
Under TiO2 loading the surface coverage of TiO2 increases from 9 to 47% with TiO2 nanoparticle size being in the range from 8.4 nm to 14 nm (Table S2). Pd nanoparticles of diameters of 4.9–6.8 nm on TiO2/f-MWCNTs cover 2.4–3.4% of the surface. Decreasing TiO2 coverage after Pd deposition, the most predominant for Pd-8%TiO2/f-MWCNTs, indicates minor content of Pd NPs on TiO2. The diameters of TiO2 and Pd NPs obtained in this procedure are closer to the values obtained from HR-TEM and larger than those resulting from XRD since the QUASES-Analyze method accounts for amorphous structure, agglomerates and possible intermetallic compounds.
Surface elemental content
The surface of TiO2 and Pd-TiO2-decorated f-MWCNTs shows Pd, Ti, C, O, traces of N and no B resulting from NaBH4 reducing agent contamination (Fig. 4, Fig. S4-Supplementary Information).
The elemental surface content (Table 1) was evaluated from the area of the XPS C 1 s, O 1 s, Ti 2p3/2–1/2, Pd 3d5/2–3/2 and N 1 s spectra (Fig. 4, Fig. S4).
The titanium oxide indicates stoichiometry of TiO2. Increasing loading of TiO2 NPs on the surface of f-MWCNTs (TiO2 of 5–60 wt.%) leads to decreasing C and increasing Ti and O content, whereas content of deposited Pd does not change (Table 1, Fig. S5a). This indicates deposition of TiO2 and Pd on the surface of f-MWCNTs and then TiO2/f-MWCNTs, respectively. For TiO2/f-MWCNTs of 5–10 wt.% TiO2 loading the surface content of Ti is consistent with that resulting from the chemical procedure and TGA in contrary to a larger TiO2 loading, where Ti surface content is nearly twice smaller, confirming TiO2 nanoparticles agglomeration shown previously by HR-TEM (Fig. 1). Pd surface content of 19.7–24.4 wt.% is in a reasonable agreement with the chemical procedure, i.e., 20 wt.%. The surface Pd/Ti ratio exceeds the respective resulting from the chemical procedure being more consistent for a larger TiO2 loading (Fig. S5b), indicating that Pd is placed majorly on TiO2 for a low TiO2 loading and majorly on f-MWCNTs for a higher TiO2 loading. Increasing loading of TiO2 leads to decreasing Pd/O ratio, increasing Ti/O ratios, decaying after Pd decoration (Fig. S5c), what suggests increasing Pd oxide content, changes of TiO2 environment and formation of chemical bonds. Increasing size of Pd and TiO2 crystallites at high TiO2 loading (Table S1) is due to aggregation of NPs at the surface of lower hydrophilicity. Formation of bonds between Pd and carbon–oxygen groups of graphene materials leading to Pd NPs size inversely proportional to the content of functional groups has been previously reported [46].
Chemical state of atoms
TiO2 standard and low TiO2 loading (5–20 wt.%) TiO2/f-MWCNTs catalysts showed a charging effect. Spectrum BE of TiO2 standard was calibrated on C 1 s at BE of 284.4 eV, whereas BE of TiO2/f-MWCNTs was calibrated on C 1 s BE of the respective Pd loaded catalyst showing C 1 s BE at about 284.4 eV.
After energy calibration of C 1 s, Ti 2p3/2–1/2, Pd 3d5/2–3/2 and O 1 s experimental spectra they were fitted to different chemical states using Gaussian–Lorentzian functions (Fig. 5 and Fig. S6). The chemical state of C, Ti, Pd, O atoms and their content were determined using the values of BE provided in the NIST database [38] and literature.
Chemical state of C atoms
The C 1 s spectrum was fitted to components reflecting different C hybridizations (C sp2, C sp3), carbon–oxygen groups (hydroxyl—C–OH, carbonyl—C=O, carboxyl—C–OOH) and C vacancy defects (Fig. S6a), assuming the values of BE shifted from the C sp2 component in the range according to the BE values reported in the literature [41, 46,47,48,49,50,51,52,53,54]. The BE of C sp2 in a perfect graphite is 284.5 eV [47]. The difference between values of BE for the sp2 and sp3 component of 0.9 eV for amorphous carbon [48], in agreement with a theory, and 0.8 eV for diamond like films [49], has been reported previously. The BE difference of 1.3–1.35 eV was reported for onion like nanodiamonds [50] and amorphous carbon film [51]. Otherwise, for graphite, oxidized graphite, graphene materials, carbon nanotubes the values of BE shift from C sp2 to C sp3 and carbon–oxygen groups were reported in the following range: 0.8–1.35 eV (C sp3), 1.3–2.5 eV (C–OH), 2.1–3.5 eV (C=O), 4.0–5.4 eV (C–OOH)] and − 0.7 to − 1 eV (C vacancy defects) [41, 46,47,48,49,50,51,52,53,54].
The values of BE for different oxygen groups reflected in C 1 s spectrum are in a range of those reported in the literature (Table 2). Increasing loading of TiO2 leads to decreasing number of carbon vacancy defects, C sp3 and oxygen groups such as C=O>C–OOH > C–OH (Fig. S7a). Simultaneously, increasing full width at half maximum (FWHM) (Fig. S7b) and decreasing BE (Fig. S7c) of C 1 s sp2 component indicate increasing disorder and number of defects in hexagonal carbon structures due to TiO2 loading [54]. This would be consistent with formation of larger Pd NPs at higher TiO2 coverages due to decreased content of centers for Pd positive ion nucleation, i.e., carbon–oxygen groups and carbon vacancy defects.
Chemical state of Pd atoms
The Pd 3d5/2–3/2 spectra recorded from Pd-TiO2/f-MWCNTs samples were fitted to components of metallic Pd, PdO and PdO2 (Fig. S6b) with BE values accounting for the reported BE values, i.e., 336.3–337.2 eV (PdO) and 337.9–339.3 eV (PdO2) with smaller BE values for respective Pd surface oxides in contrary to bulk oxides [38, 55]. The investigated samples indicate metallic Pd (335.2–335.5 eV), PdO (336.0–337.6 eV) and PdO2 (337.4–338.2 eV) (Table 3). The obtained values of BE and FWHM for Pd are larger than those for metallic Pd, i.e., 335.1 eV and 1.3. eV, respectively, confirming the presence of Pd nanoparticles of increasing size under TiO2 loading since as reported by Wertheim et al. [56] for Pd clusters a positive shift of the BE values, accompanied by increasing FWHM, indicates decreasing cluster size due to the unfilled 4d valence band and d-band narrowing. This confirms the results of HR-TEM, XRD and XPS-QUASES.
The ratio of Pd to palladium oxides decreasing under TiO2 loading (Table 3) indicates larger amount of metallic Pd at low TiO2 loading (4–8 wt.%). Simultaneously, the content of PdO2 exceeds that of PdO. This remains consistent with the results of quantitative analysis indicating decreasing ratio of Pd to O (Fig. S5c). The BEs of PdO and PdO2 decrease systematically with TiO2 loading indicating surface oxides, what may suggest formation of Pd–O-C and Pd–O-Ti bonds. This effect is stronger at higher TiO2 loading (Pd nanoparticles are placed majorly on f-MWCNTs) than at low TiO2 loading (Pd nanoparticles are placed majorly on TiO2) (Fig. S5b, Table 3).
Chemical state of Ti atoms
The energy calibrated and fitted Ti 2p3/2–1/2 spectra (analyzer acquisition parameter k = 4) (Fig. 5, S6) show differences in BE, FWHM and doublet splitting (DS) values under TiO2 loading and after depositing Pd (Fig. 6a, b). The oxygen content resulting from the quantitative analysis and Ti amount (Table 1) assuming stoichiometry of TiO2, carbon–oxygen groups (Table 2) and Pd oxides (Table 3) indicates a reasonable agreement and verifies the stoichiometry of TiO2 for TiO2/f-MWCNTs and respective Pd-loaded samples (Fig. S8a-b) in nearly the whole range of loading. Discrepancies for TiO2/f-MWCNTs may be explained by the presence of oxygen groups on TiO2 nanoparticles not considered in evaluation. Literature reports DS value of 5.7–5.76 eV for TiO2 and 6.09–6.15 eV for Ti [38]. Under TiO2 loading DSs increase from 5.5 to 5.7 eV, whereas BEs and FWHMs decrease due to modification of TiO2 stoichiometry and formation of bonds with f-MWCNTs’ carbon–oxygen groups. After Pd deposition the further decrease of DSs, BEs and FWHMs, especially at low TiO2 loadings, suggests formation of Pd-TiO2 interface.
The Ti 2p3/2–1/2 spectra (analyzer acquisition parameter k = 16) (Fig. 5, S6) were fitted to Gaussian–Lorentzian functions for quantification of oxygen vacancies, which are created under reduction conditions of synthesis (microwaves, NaBH4). When Ti4+ is reduced to Ti3+, one Ti–O bond of Ti–O–Ti is broken, generating oxygen vacancies (Ti–O• + O vacancy–Ti3+) [57]. The end of Ti–O• may be bonded with H atom of the reductant NaBH4 and reaching the equilibrium form Ti − OH [57]. Thus, the availability of Ti3+ is accompanied by the availability of Ti − OH. The NIST XPS database provides the BEs of Ti 2p3/2–1/2 for TiO2 (Ti4+) in the range of 458.2–459.6 eV and for TiO2 anatase of 458.6–458.8 eV [38]. Results of Ti 2p3/2–1/2 spectra fitting indicate Ti4+, Ti3+ (oxygen vacancy) and TiOx/Ti [19, 38] chemical states of averaged BEs over all the samples (Table 4). According to the literature [38] the BEs for TiO0.73–TiO1.5 are shifted to lower values (454.6–456.8 eV), for TiOx/Ti to higher values (about 460.2 eV), whereas for intermetallic Ti oxide and other metal/metal oxides (Fe, Zr, Ni) the BEs overlap with those of TiO2 (458.2–458.7 eV). Increasing loading of TiO2 on f-MWCNTs leads to decreasing content of oxygen vacancies with the ratio of Ti+4/Ti+3 of 2.1–6.7 (Table 4). Deposition of Pd decreases the respective ratio for each sample of specific TiO2 loading. The largest difference is observed for Pd-8%TiO2/f-MWCNTs, i.e., from 4.7 to 1.7, whereas for other samples this difference is less remarkable, suggesting that Pd is deposited on oxygen vacancies. Simultaneously, the BEs shift toward smaller values confirms formation of Pd-TiO2 interface.
Chemical state of O atoms
The O 1 s of TiO2 and all Pd-TiO2/f-MWCNTs samples spectra were fitted to components attributed to TiO2 [19], C = O, C–OH [46] and overlapping PdO and PdO2 chemical forms [55] (Fig. 5, S6). The reasonable quantitative agreement for oxygen groups from TiO2 (Ti quantification from Table 1), Pd oxides (PdO and PdO2 quantification from Table 3) and carbon–oxygen groups (O quantification from Table 2 including water) was obtained.
Auger spectra and Auger parameters
The Auger spectra of titanium. palladium and oxygen (Ti LMM, Pd MNN and O KLL) reflecting the density of valence band states show the lineshape and intensity changes after depositing TiO2 and then Pd on f-MWCNTs (Fig. 7). The increased density of valence band states of Ti L23M23M45, Pd M5N45N45 and O KL23L23 confirms the formation of bonds between f-MWCNTs, TiO2 and Pd/PdO, i.e., Ti–O–C, Pd–O–Ti and Pd–O–C (Fig. S9a-c).
The Auger parameters (APs) describe the electronic structure at surface and interface such as a local electronic charge redistribution and transfer at an atomic level determining the AP values independent of the charging effects both for insulating and semiconducting materials. The APs formulated previously [58] are defined as the final state Auger parameter, α, and the initial state Auger parameter, β, i.e., α = EA + EB and β = EA + 3EB, where EA and EB are kinetic energy (KE) of Auger electron transition and BE of photoelectron transition. These formulas consider kinetic and binding energy scale together, and therefore, the charging effect is compensated. Changes in Auger parameter between two environments such as element and its other chemical state defined as differences between Auger parameters α and β, i.e., Δα = αmet-αenv = 2ΔR and Δβ = βmet-βenv = 2ΔV [58], indicate the difference between atomic potential in the core and the core hole screening efficiency (extra-atomic relaxation) and between atomic potentials in the core of the atom at the atomic site, respectively. The final state effect (Δα) refers to a shift in polarization energy at the core-ionized atom and considers the charge transfer in a local valence band orbital of the core-ionized atom contributing to the electronic relaxation energy of all the other atoms in the system. The initial state effect (Δβ) represents a chemical shift as a result of a ground state electronic structure and depends on the bonding to neighboring atomic valence states relating to electronic states, e.g., band structures, bond directionality and structural parameters like atomic positions and Madelung constants of the bonded atom.
Depositing TiO2 and Pd on f-MWCNTs and variation of TiO2 content modifies the Auger parameters α and β evaluated for Ti (AP-L23M23M45—Ti 2p3/2). (b) Pd (AP-M5N45N45-Pd 3d5/2) and O (AP-O Kl23L23-O 1 s) (Fig. 8 and Table S4). Increasing TiO2 NPs loading on f-MWCNTs and then depositing Pd NPs leads to changes of APs’ indicating an electron charge transfer and atomic coordination and positions and therefore formation of bonds between positively charged states with negatively charged hydrolyzed carbon–oxygen groups, carbon vacancy defects, oxygen vacancies (Fig. 8, Table S4). These changes of APs are the most significant at low TiO2 coverages (5–20 wt.%). Negative value of Δα(Ti) of TiO2 NPs on f-MWCNTs, increasing with TiO2 content, indicates charge transfer from TiO2 to f-MWCNTs, larger values of Δα(Pd) for Pd NPs on TiO2/f-MWCNTs suggest charge transfer to Pd, whereas Δα(O) positive values confirm charge transfer to f-MWCNTs (Table S4). The most remarkable changes of Δβ(Ti), Δβ(Pd) and Δβ(O) values for low TiO2 coverages, especially for Pd-8%TiO2/f-MWCNTs, result from Pd-TiO2 formed at oxygen vacancies (Table S4).
The obtained results are consistent revealing the presence of Pd-TiO2-f-MWCNTs interfaces with a charge transfer from TiO2 to f-MWCNTs and from TiO2/f-MWCNTs to Pd, and therefore electron-rich surface of catalyst and support, where modification of structural, chemical and electronic properties proceeds via increasing TiO2 loading. At low TiO2 loading (5–20 wt.%), of a larger number of TiO2 oxygen vacancies Pd is deposited on oxygen vacancies forming Pd-TiO2 interface. Changes in features of Pd, Ti, O XPS and XAES spectra such as BEs, FWHMs, DSs, density of valence states and Auger parameters indicate formation of a larger number of oxygen vacancies at low TiO2 coverages, Pd deposition majorly on oxygen vacancies and stronger Pd-TiO2-f-MWCNTs interfaces via Ti–O-C, Pd–O-Ti and Pd–O-C bonds resulting from electron transfer from TiO2 to f-MWCNTs and to Pd. Such Pd-carbon materials [46], TiO2-MWCNTs [59] and Pd-TiO2 [60] have been already reported. The extended X-ray absorption fine structure spectroscopy of Pd/Ce1-xTixO2 system provided also an evidence on existence of Pd-Ti bonds [61].
Fuel cell and electrochemical tests
The Pd-TiO2/f-MWCNTs catalysts show values of specific activity in the range of 39.7–106.2 mW mgPd−1 and stability in the range of 8–240 h dependent on TiO2 content (Fig. 9). Such high values of stability have not been reported in the literature. For the respective TiO2 the specific activity decreases in the following order:
Comparison of maximum specific power versus current (left panel) and catalytic stability at a constant specific current (40 mA mgPd−1) (right panel) for Pd-TiO2/f-MWCNTs catalysts on the anode side (reaction of a formic acid electrooxidation) and 60%Pt/C on the cathode side. The sample notation is described in Sect. "Samples and preparation".
Pd-8%TiO2 > Pd-4%TiO2 > Pd-16%TiO2 > Pd-32%TiO2 > Pd-48%TiO2, whereas stability in the order:
Pd-8%TiO2(240 h) > Pd-16%TiO2(100 h) > Pd-4%TiO2(25 h) > Pd-32%TiO2 ~ Pd-48%TiO2 (8 h).
Stability (durability) increases with specific activity. Therefore, these two parameters increase with decreasing crystallite size, Ti and O atomic content and Pd/PdOx ratio, being the highest for Ti/Pd content ratio of about 1 and below, as reported elsewhere [20]. Similarly, activity and stability increase proportionally to the number of C vacancy defects, the carbon oxygen groups and the ratio of Ti4+/Ti3+, electron charge transfer in Pd, Ti and O atoms and modification of configuration, indicating formation of Pd-TiO2 interface. The catalyst with 8 wt.% of TiO2 is the most active and stable in FAOR during fuel cell operation. Closer stability of Pd-8wt.% > Pd-16wt.% can be explained with similar electronic properties and charge transfer in the system (Table S4). Both Pd-8wt.% and Pd-16wt.% catalysts exhibit spontaneous oscillations of the fuel cell potential. These oscillations may be explained either as: (i) electrooxidation of impurities of the formic acid to COads strongly adsorbed on Pd surface causing decrease of FC voltage and power, although at sufficiently low voltage the rate of oxidation of COads to CO2 increases cleaning the surface of the catalyst what results in an increase of FC voltage and power [17] or (ii) direct electrooxidation of formic acid to CO2, which is periodically removed from the catalyst layer [8]. The prepared catalysts were found to be more resistant to deactivation in the direct formic acid fuel cell than the previously reported 20 wt.% Pd/f-MWCNTs [40] and 20 wt.% Pd/ZrO2/f-MWCNTs [17] showing an excellent long-term performance.
The stability of catalysts is predominantly attributed to the self-cleaning mechanisms of the supported Pd catalyst, related to its electronic properties, where many processes are involved into removal of adsorbed CO and hydrogen from the surface. These processes are governed predominantly by electron transport properties in a system exhibiting a strong metal–support interaction weakening the Pd-COads bond, facilitating intermediate oxidation–reduction reactions, CO and hydrogen spillover, and by the presence of oxygen available from –OH groups from oxides and functionalized carbon nanomaterials [9, 15,16,17, 19,20,21,22,23,24,25,26,27]. As reported recently [60] the dynamic evolution of multi-Pdn+ states (Pd1+, Pd2+ − O2− and Pd3+) stabilized on the support and formed at the interface of Pd NPs with TiO2 under conditions of formic acid dehydrogenation, acting as electron and hydride acceptors is majorly responsible for a high catalytic activity and stability.
The cyclic voltammograms obtained in 0.1 M HClO4 are typical for Pd-based catalysts (Fig. 10). There are three main regions: (1) in the range of − 0.2–0.1 V vs. Ag/AgCl, where hydrogen adsorption and desorption occurs, (2) above 0.3 V, where palladium oxidation and reduction of the formed oxide occurs, (3) in the range 0.1–0.3 V, where the double-layer region occurs. Usually, the area of the peaks in the first two regions can be used to determine the electrochemical active surface area (ECSA) of the Pd-based catalyst. From Fig. 10 the highest currents obtained for these regions are for Pd/f-MWCNTs sample. For all the catalysts containing TiO2 much smaller currents are observed, which indicates that Pd is not oxidized as strongly as the Pd/f-MWCNTs sample of the same amount of palladium. Palladium oxide is non-active in HCOOH oxidation, so it is better to avoid it.
Activities of different Pd/f-MWCNTs and Pd-TiO2/f-MWCNTs catalysts in 0.1 M HClO4 + 3 M HCOOH solution show the higher values for the samples containing TiO2 nanoparticles (Fig. S10). Also, for measurements performed at 10 mV/s samples behave differently (Fig. 11). For Pd/f-MWCNTs sample the oxidation peak (around 0.256 V vs. Ag/AgCl) of HCOOH in the forward scan is higher than the peak in the reverse scan (around 0.364 V vs. Ag/AgCl). The value of the potential at which the oxidation occurs suggests that the reaction follows mainly the direct oxidation pathway. For the catalysts with TiO2 the shape of the curve changes. In the forward scan, the oxidation peak shifts (0.316 V vs. Ag/AgCl for Pd-4%TiO2, 0.198 V vs. Ag/AgCl for both Pd-8%TiO2 and Pd-16%TiO2, 0.245 V vs. Ag/AgCl for Pd-32%TiO2) and an additional peak at higher potentials can be distinguished. Lower potentials could be beneficial for the catalyst activity. The largest difference occurs in the backward scan, where peaks from CO reduction can be detected (especially for Pd-4%TiO2, Pd-16%TiO2). Furthermore, in the reverse scan, TiO2-containing catalysts generally exhibit significantly higher currents. In such cases, increased currents are typically noted during the reverse scan due to the blocking of active sites during the forward potential scan with reaction intermediates (such as adsorbed CO) formed at lower potentials. These intermediates are then oxidized at higher potentials, resulting in the cleaning of the catalyst surface, and improving the oxidation of formic acid.
The impact of temperature on the electro-oxidation of formic acid in DFAFC investigated using a linear sweep voltammetry experiments and conducted at various temperatures ranging from 30 to 70 °C is shown in Fig. S11. The most notable observation is the increase in the current density with a rising temperature. This phenomenon can be attributed to the temperature-dependent activation of the electrooxidation process of formic acid. However, it is important to note that the oxygen reduction reaction is also thermally activated, which typically results in an elevation of cell voltage at higher temperatures. The maximum current density of the samples exhibits an upward trend with increasing temperature in the range from 30 to 70 °C, while the overpotential shows a decreasing trend, particularly noticeable between 30 and 40 °C.
Conclusions
Pd deposited on TiO2 supported on f-MWCNTs catalysts was investigated as novel electrocatalysts on the anode side for formic acid electrooxidation. TiO2 NPs on f-MWCNTs surface of different TiO2 content varying from 5 to 60 wt.% were obtained using the microwave-assisted hydrothermal method, whereas 20 wt.% of Pd NPs were deposited on TiO2/f-MWCNTs nanocomposites through reduction of palladium (II) chloride salt with sodium borohydride.
The structure and chemical composition of the prepared catalysts were characterized by XRD, TGA, STEM, HR-TEM, XPS and XAES. Composition of catalysts confirmed by XPS and TGA was close to that resulting from a chemical procedure. MWCNTs support functional groups such as –OH, C=O, C–OOH and carbon vacancy defects lead to formation of TiO2 and Pd crystallites of size of 3–4 nm increasing with TiO2 loading due to decreasing surface hydrophilicity. TiO2 on f-MWCNTs shows oxygen vacancy defects decreasing with the loading. At low TiO2 coverage (5–20 wt.%) Pd is deposited on oxygen vacancy defects and then majorly at TiO2 NPs. For larger loading with decreasing oxygen vacancies and increasing Pd oxide content Pd NPs are deposited majorly at the surface of f-MWCNTs. Changes in the features of XPS and XAES spectra of Pd, Ti, O such as intensity ratios, BEs, FWHMs, DSs, density of valence states and Auger parameters indicate formation of Pd-TiO2-f-MWCNTs interfaces via Ti–O–C, Pd–O-Ti and Pd–O–C bonds and electron transfer from TiO2 to f-MWCNTs and to Pd.
The 20 wt.% Pd-8 wt.% TiO2-72 wt.% f-MWCNTs catalyst exhibited the highest initial specific activity (106.2 mW mgPd−1) and stability (240 h) at operating conditions attributed to: (i) formation of strong intermetallic Pd-TiO2 weakening the bond with adsorbed CO and facilitating hydrogen and CO spillover, (ii) access to oxygen to oxidize COads to CO2 by –OH groups available from TiO2 and f-MWCNTs, (iii) strong metal–support interaction facilitating electron transport and (iv) small Pd NPs size of a high active surface area. Dynamic evolution of multi Pdn+ states (Pd1+, Pd2+ − O2− and Pd3+) stabilized on the support and formed at the interface of Pd NPs with TiO2 under conditions of formic acid dehydrogenation, acting as electron and hydride acceptors is majorly responsible for a high catalytic activity and stability. The electrochemical tests confirm the DFAFC results, where the highest durability of catalysts is related to the lowest value of oxidation potential indicating the best resistance to CO poisoning.
Data availability
The data are available in the manuscript and Supplementary Information files. Additional data can be obtained from the corresponding authors upon a reasonable request.
Code availability
DOI of the published manuscript and the Supplementary Information is given.
References
Yu X, Pickup PG (2008) Recent advances in direct formic acid fuel cells (DFAFC). J Power Sources 82(1):124–132. https://doi.org/10.1016/j.jpowsour.2008.03.075
Rees NV, Compton RG (2011) Sustainable energy: A review of formic acid electrochemical fuel cells. J Solid State Electrochem 15(10):2095–2100. https://doi.org/10.1007/s10008-011-1398-4
Hossain SKS, Ahmad Alwi MM, Saleem J, AlOdail F, Basu A, Hossai MM (2022) Recent advances in anode electrocatalysts for direct formic acid fuel cell-II-Platinum-based catalysts. Chem Rec 22:e202200156. https://doi.org/10.1002/tcr.202200156
Nogalska A, Navarro AB, Garcia-Valls R (2020) Mea preparation for direct formate/formic acid fuel cell—comparison of palladium black and palladium supported on activated carbon performance on power generation in passive fuel cell. Membranes (Basel) 10(11):1–10. https://doi.org/10.3390/membranes10110355
Antolini E (2009) Palladium in fuel cell catalysis. Energy Environ Sci 2(9):915–931. https://doi.org/10.1039/b820837a
Arenz M, Stamenkovic V, Schmidt TJ, Wandelt K, Ross PN, Markovic NM (2003) The electro-oxidation of formic acid on Pt-Pd single crystal bimetallic surfaces. Phys Chem Chem Phys 5(19):4242–4251. https://doi.org/10.1039/b306307k
Law WL, Platt AM, Wimalaratne PDC, Blair SL (2009) Effect of organic impurities on the performance of direct formic acid fuel cells. J Electrochem Soc 156(5):B553. https://doi.org/10.1149/1.3080691
Mikołajczuk A, Borodzinski A, Kedzierzawski P, Stobinski L, Mierzwa B, Dziura R (2011) Deactivation of carbon supported palladium catalyst in direct formic acid fuel cell. Appl Surf Sci 257(19):8211–8214. https://doi.org/10.1016/j.apsusc.2011.04.078
Malolepszy A, Mazurkiewicz M, Mikolajczuk A, Stobinski L, Borodzinski A, Mierzwa B, Lesiak B, Zemek J, Jiricek P (2011) Influence of Pd-Au/MWCNTs surface treatment on catalytic activity in the formic acid electrooxidation. Phys Status Solidi Curr Top Solid State Phys 8(11–12):3195–3199. https://doi.org/10.1002/pssc.201100215
Yu X, Pickup PG (2009) Novel Pd-Pb/C bimetallic catalysts for direct formic acid fuel cells. J Power Sources 192(2):279–284. https://doi.org/10.1016/j.jpowsour.2009.03.036
Yu X, Pickup PG (2010) Deactivation resistant PdSb/C catalysts for direct formic acid fuel cells. Electrochem Commun 12(6):800–803. https://doi.org/10.1016/j.elecom.2010.03.037
Bauskar AS, Rice CA (2013) Spontaneously Bi decorated carbon supported Pd nanoparticles for formic acid electro-oxidation. Electrochim Acta 107:562–568. https://doi.org/10.1016/j.electacta.2013.06.042
Chen CH, Chiou YJ, Liou WJ, Lin LWS, HM, Wu SH, Borodziński A, Kedzierzawski P, Stobinski L, Chien SH, (2011) Synthesis and electrocatalysis application of hybrid platinum/cerium oxide/multi-walled carbon nanotubes. Funct Mater Lett 4(3):295–298. https://doi.org/10.1142/S1793604711002032
Matos J, Borodzinski A, Zychora AM, Kedzierzawski P, Mierzwa B, Juchniewicz K, Mazurkiewicz M, Hernández-Garrido JC (2015) Direct formic acid fuel cells on Pd catalysts supported on hybrid TiO2-C materials. Appl Catal B Environ 163:167–178. https://doi.org/10.1016/j.apcatb.2014.07.063
Tseung ACC, Chen KY (1997) Hydrogen spill-over effect on Pt/WO3 anode catalysts. Catal Today 38(4):439–443. https://doi.org/10.1016/S0920-5861(97)00053-9
Qu WL, Wang ZB, Sui XL, Gu DM, Yin GP (2013) ZrC-C and ZrO2-C as novel supports of Pd catalysts for formic acid electrooxidation. Fuel Cells 13(2):149–157. https://doi.org/10.1002/fuce.201200204
Malolepszy A, Mazurkiewicz M, Stobinski L, Lesiak B, Kövér L, Tóth J, Mierzwa B, Borodzinski A, Nitze F, Wågberg T (2015) Deactivation resistant Pd-ZrO2 supported on multiwall carbon nanotubes catalyst for direct formic acid fuel cells. Int J Hydrogen Energy 40(46):16724–16733. https://doi.org/10.1016/j.ijhydene.2015.08.048
Tauster SJ, Fung S, Garten RL (1978) Strong metal-support interactions. Group 8 Noble Metals Supported on TiO2. J Am Chem Soc 1(100):170–175. https://doi.org/10.1021/ja00469a029
Shi W, Park AH, Xu S, Yoo PJ, Kwon YU (2021) Continuous and conformal thin TiO2-coating on carbon support makes Pd nanoparticles highly efficient and durable electrocatalyst. Appl Catal B Environ 284:119715. https://doi.org/10.1016/j.apcatb.2020.119715
Wan H, Dong Q, Zhu G, Yu G, Yin T, Huang M (2015) Synthesis of Pd/TiO2-C composite catalysts and investigation of its performance for the electrooxidation of formic acid. Int J Hydrogen Energy 40(41):14179–14186. https://doi.org/10.1016/j.ijhydene.2015.08.081
Chen J, Gao X, Wu Y, Ren Y, Kang Y, Li Y, Yue B, He H, Chen X (2023) Facile synthesis of the TiO2-supported ultrathin palladium facet composite catalyst with superior metal dispersion and enhanced catalytic performance in formic acid electro-oxidation. J Phys Chem C 127:22608–22617. https://doi.org/10.1021/acs.jpcc.3c05482
Yang P, Wei L, Xiao X, Zhou Z, Li J, Zhang Y, Xie Y, Yang N, Fei J (2022) Electrocatalytic oxidation of formic acid on Pd/CNTs nanocatalysts synthesized in special “non-aqueous” system. J Electroanal Chem 906:115980. https://doi.org/10.1016/j.jelechem.2021.115980
Yang P, Zhang L, Wei X, Dong S, Ouyang Y (2022) Pd3Co1 alloy nanocluster on the MWCNT catalyst for efficient formic acid electro-oxidation. Nanomaterials 12:4182. https://doi.org/10.3390/nano12234182
Ramli ZAC, Pasupuleti J, Saharuddin TST, Yusoff YN, Roslam WN, Isahak W, Baharudin L, Yaw CT, Koh SP, Kiong ST (2023) Electrocatalytic activities of platinum and palladium catalysts for enhancement of direct formic acid fuel cells: an updated progress. Alex Eng J 76:701–733. https://doi.org/10.1016/j.aej.2023.06.069
Hossain SKS, Alwi MM, Saleem J, Al-Hashem HT, McKay G, Mansour S, Ali SS (2021) Bimetallic Pd-Co nanoparticles supported on nitrogen-doped reduced graphene oxide as efficient electrocatalysts for formic acid electrooxidation. Catalysts 11:910. https://doi.org/10.3390/catal11080910
Li C, Tang Z, Shi L, Li Y, Ji Y, Zhang K, Yang Z, Yan Y-M (2023) Constructing a triangle ensemble of Pt clusters for enhanced direct-pathway electrocatalysis of formic acid oxidation. Chemistry 5:1621–1633. https://doi.org/10.3390/chemistry5030111
Ortega-Murcia A, Navlani-Garcia M, Morallón E, Cazorla-Amorós D (2020) MWCNTs-supported PVP-capped Pd nanoparticles as efficient catalysts for the dehydrogenation of formic acid. Front Chem 8:359. https://doi.org/10.3389/fchem.2020.00359
Rice C, Ha S, Masel RI, Wieckowski A (2003) Catalysts for direct formic acid fuel cells. J Power Sources 115(2):229–235. https://doi.org/10.1016/S0378-7753(03)00026-0
Brewer L, Wengert PR (1973) Transition metal alloys of extraordinary stability; an example of generalized lewis-acid-base interactions in metallic systems. Metall Trans 4(11):2674. https://doi.org/10.1007/BF02644280
Hammer B, Nørskov JK (2000) Theoretical surface science and catalysis—calculations and concepts. Adv Catal 45:71–129. https://doi.org/10.1016/S0360-0564(02)45013-4
Yang P, Zhang L, Wei X, Dong S, Quyang Y (2022) Pd3Co1 alloy nanocluster on the MWCNT catalysts for efficient formic acid electro-oxidation. Nanomaterials 12:4182. https://doi.org/10.3390/nano12234182
Wang C, Liu X, Wang J, Li Y, Xie S, Liu F, Zhang C, Zheng Y, Shan W, He H (2024) Tuning SIMS to stabilize metallic Pd species: a case on Pd/TiO2 for HCHO oxidation. Chin Chem Lett 35:108739. https://doi.org/10.1016/j.cclet.2023.108739
Liu H, Yuan C, Wu S, Sun C, Huang Z, Xu H, Shen W (2023) Constructing an oxygen vacancy- and hydroxyl-rich TiO2- supported Pd catalyst with improved Pd dispersion and catalytic stability. J Chem Phys 159:124701. https://doi.org/10.1063/5.0171023
Tang Z, Li Y, Zhang K, Wang X, Wang S, Sun Y, Zhang H, Li S, Wang J, Gao X, Hou Z, Shi L, Yuan Z, Nie K, Xie J, Yang Z, Yan Y-M (2023) Interfacial hydrogen spillover on Pd-TiO2 with oxygen vacancies poromotes formate electrooxidation. ACS Energy Lett 8:3945–3954. https://doi.org/10.1021/acsenergylett.3c01426
Stobinski L, Lesiak B, Kövér L, Tóth J, Biniak S, Trykowski G, Judek J (2010) Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J Alloys Compd 501(1):77–84. https://doi.org/10.1016/j.jallcom.2010.04.032
Kover L, Varga D, Cserny I, Toth J, Tokesi K (1992) Some applications of high-energy. high-resolution auger electron spectroscopy using bremsstrahlung radiation. Surf Interface Anal 9:9–15. https://doi.org/10.1002/sia.740190106
Crist V, The International XPS Database of XPS Reference Spectra. The XPS Library. Vincent Crist 2023. https://xpsdatabase.com.
Naumkin AV, Kraut-Vass A, Gaarenstroom W, Powell CJ, NIST X- Ray Photoelectron Database. NIST SRD 20 ver. 4.1 online PC Gaithersburg: NIST. U.S. Department of Commerce. Published online 2012:2012. Accessed August 2023.
Nitze F, Mazurkiewicz M, Malolepszy A, Mikolajczuk A, Kędzierzawski P, Tai C-W, Hu G, Kurzydłowski KJ, Stobinski L, Borodzinski A, Borodzinski A, Wagberg T (2012) Synthesis of palladium nanoparticles decorated helical carbon nanofiber as highly active anodic catalyst for direct formic acid fuel cells. Electrochim Acta 63:323–328. https://doi.org/10.1016/j.electacta.2011.12.104
Mikolajczuk A, Borodzinski A, Stobinski L, Kedzierzawski P, Lesiak B, Kövér L, Tóth J, Lin HM (2010) Physicochemical characterization of the Pd/MWCNTs catalysts for fuel cell applications. Phys Status Solidi Basic Res 247(11–12):3063–3067. https://doi.org/10.1002/pssb.201000242
Stobinski L, Lesiak B, Zemek J, Jiricek P, Biniak S, Trykowski G (2010) Studies of oxidized carbon nanotubes in temperature range RT-630°C by the infrared and electron spectroscopies. J Alloys Compounds 505:379–384. https://doi.org/10.1016/j.jalicom
Gao B, Chen GZ, Puma GL (2009) Carbon nanotubes/titanium dioxide (CNTs/TiO2) nanocomposites prepared by conventional and novel surfactants wrapping sol-gel method exhibiting enhanced photocatalytic activity. App Catal B: Environ 89:504–509. https://doi.org/10.1016/j.apcatb.2009.02.009
Ortiz-Ortega E, Carrera-Carritos R, Arjona R, Guerra-Balcázar M, Cuevas-Muniz FM, Arriega LG, Ledesma-Garcia J (2014) Pd nanostructures with high tolerance to CO poisoning in the formic acid electrooxidation reaction. Proc Chem 12:9–18. https://doi.org/10.1016/j.proche.2014.12.035
Touggard S, QUASES – Quantitative Analysis of Surfaces by Electron Spectroscopy. Software for Quantitative XPS/AES of Surface Nano-Structures by Analysis of the Peak Shape and Background. Version 5.0; 1994–2002 QUASES Tougaard ApS; http://www.quases.com.
QUASES-IMFP-TPP2M Inelastic electron mean free path calculated from Tanuma. Powell and Penn TPP2M formula. Quases-Tougaard Inc.. 2000–2010. http://www.quases.com
Lesiak B, Jiricek P, Bieloshapka I (2017) Chemical and structural properties of Pd nanoparticles-decorated graphene- electron spectroscopic methods by QUASES. Appl Surf Sci 404:300–309. https://doi.org/10.1016/j.apsusc.2017.01.183
Prince KC, Ulrych I, Peloi M, Ressel B, Cháb V, Crotti C, Comicioli C (2000) Core-level photoemission from graphite. Phys Rev B Condens Matter Mater Phys 62(11):6866–6868. https://doi.org/10.1103/PhysRevB.62.6866
Haerle R, Riedo E, Pasquarello A, Baldereschi A (2002) C sp2/sp3 hybridization ratio in amorphous carbon from C core-level shifts: X-ray photoelectron spectroscopy and first-principles calculation. Phys Rev B Condens Matter Mater Phys 65(4):045101. https://doi.org/10.1103/PhysRevB.65.045101
Mérel P, Tabbal M, Chaker M, Moisa S, Margot J (1998) Direct evaluation of the sp3 content in diamond-like-carbon films by XPS. Appl Surf Sci 136(1–2):105–110. https://doi.org/10.1016/S0169-4332(98)00319-5
Butenko YV, Krishnamurthy S, Chakraborty AK, Kuznetsov VL, Dhanak VR, Hunt MRC, Šiller L (2005) Photoemission study of onionlike carbons produced by annealing nanodiamonds. Phys Rev B Condens Matter Mater Phys 71(7):1–10. https://doi.org/10.1103/PhysRevB.71.075420
Taki Y, Takai O (1998) XPS structural characterization of hydrogenated amorphous carbon thin films prepared by shielded arc ion plating. Thin Solid Films 316(1–2):45–50. https://doi.org/10.1016/S0040-6090(98)00386-1
Stobinski L, Lesiak B, Malolepszy A, Mazurkiewicz M, Mierzwa B, Zemek J, Jiricek P, Bieloshapka I (2014) Graphene oxide and reduced graphene oxide studied by the XRD. TEM and electron spectroscopy methods. J Electron Spectrosc Relat Phenomena 195:145–154. https://doi.org/10.1016/j.elspec.2014.07.003
Lesiak B, Kövér L, Töth J, Zemek J, Jiricek P, Kromka A, Rangam N (2018) Csp2/sp3 hybridisations in carbon nanomaterials—XPS and (X)AES study. Appl Surf Sci 452:223–231. https://doi.org/10.1016/j.apsusc.2018.04.269
Barinov A, Gregoratti L, Dudin P, La Rosa S, Kiskinova M (2009) Imaging and spectroscopy of multiwalled carbon nanotubes during oxidation: defects and oxygen bonding. Adv Mater 21(19):1916–1920. https://doi.org/10.1002/adma.200803003
Kibis LS, Titkov AI, Stadnichenko AI, Koscheev SV, Boronin AI (2009) X-ray photoelectron spectroscopy study of Pd oxidation by RF discharge in oxygen. Appl Surf Sci 255(22):9248–9254. https://doi.org/10.1016/j.apsusc.2009.07.011
Wertheim GK, DiCenzo SB, Buchanan DNE (1986) Noble- and transition-metal clusters: the d bands of silver and palladium. Phys Rev B 33(8):5384–5390. https://doi.org/10.1103/PhysRevB.33.5384
Maimaiti H, Xu B, Sun J-Y, Feng L-R (2021) Photocatalytic synthesis of urea CO2/N2/H2O) on coal-based carbon nanotubes with the Fe-core-supported Ti3+-TiO2 composite catalyst. ACS Sustain Chem Eng 9:6991–7002. https://doi.org/10.1021/acssuschemeng.1c00644
Moretti G (1998) Auger parameter and Wagner plot in the characterization of chemical states by X-ray photoelectron spectroscopy: a review. J Electron Spectrosc Rel Phenom 95:95–144. https://doi.org/10.1016/S0368-2048(98)00249-7
Ghartavol HM, Mohammadi MR, Afshara A, Li Y (2019) On the assessment of incorporation of CNT–TiO2 core–shell structures into nanoparticle TiO2 photoanodes in dye-sensitized solar cells. Photochem Photobiol Sci 18:1840–1850. https://doi.org/10.1039/c9pp00100j
Stathi P, Belles L, Deligiannakis Y (2022) Multipotent atomic palladium species Pd1+, Pd2+−O2 −, and Pd3+ formed at the interface of Pd/TiO2 nanoparticles: electron paramagnetic resonance study. J Phys Chem C 126:14125–14137. https://doi.org/10.1021/acs.jpcc.2c02687
Hegde MS, Baidya T, Priolkar KR, Sarode PR, Asakura K, Tateno G, Koike Y (2008) Local structure of Pt and Pd ions in Ce1-x Tix O2: X-ray diffraction. X-ray photoelectron spectroscopy and extended x-ray absorption fine structure. J Chem Phys. https://doi.org/10.1063/1.2841365
Acknowledgements
The authors (A.M., M.M-P., L.S.) acknowledge the ENERGYTECH-1 project granted by Warsaw University of Technology under the program Excellence Initiative Research University (ID-UB) 1820/29/Z01/POB7/2020. The authors (L. K., J. T., B. L-O.) would like to acknowledge the support of the project between the Polish Academy of Sciences and the Hungarian Academy of Sciences (2020-2023). The author (J. T.) acknowledges the support by the European Regional Development Fund and Hungary in the frame of the project GINOP-2.2.1-15-2016-00012. The authors (A. M., M.M-P.) acknowledge the support by the Warsaw University of Technology under the program Excellence Initiative: Research University (ID-UB) MAT-Lab-1 1820/123/Z01/POB5/2022.
Author information
Authors and Affiliations
Contributions
L.S., A.M., M.M-P., B.L-O. contributed to the conceptualization. A.M., J.T., M.M-P., B.M., B.L-O. contributed to investigation, data curation, formal analysis. A.M., B.L-O. contributed to validation, visualization and writing—original draft. L.S. contributed to supervision. B.M., J.T., L.K., L.S., Y.C.C. contributed to writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in preparing this article.
Ethical approval
Ethical approval was not required for this research.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Malolepszy, A., Mazurkiewicz-Pawlicka, M., Stobinski, L. et al. Influence of TiO2 coverage on activity and stability of Pd-TiO2/MWCNT-supported catalysts used in direct formic acid fuel cells. J Mater Sci 59, 6894–6915 (2024). https://doi.org/10.1007/s10853-024-09586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09586-5