Abstract
Electrochemical 3D printing technology built on computer numerical control platforms has enabled multi-dimensional and multi-scale manufacturing of various metal materials through layered electrochemical deposition. Compared to thermal 3D printing technology, electrolyte meniscus-confined 3D printing can manufacture Cu microstructures with fewer defects and smoother surfaces. In the meantime, it is still susceptible to unstable liquid–solid-air interfaces, low deposition rates, and limited printing geometry. This work combined jet electrochemical deposition with a portable 3-axis platform to develop a cyclic high-speed electrolyte jet (HSEJ) 3D printer. It offers a faster deposition rate of 53.4 µm/h when printing ultrasmooth Cu microelectrodes with surface average roughness down to 1.1 nm and microhardness of 3.3 GPa which is much higher than the best result of 2.4 GPa obtained by the other ECD methods. It is identified that the fluctuation of cathode current density plays a crucial role in defining the nucleation morphology on the Cu surface, while the cathode current efficiency is a reliable indicator to assess the deposition localization by reflecting the variation of diffusion percentage. HSEJ 3D printing provides a sustainable pathway for the facile recycling of waste cables into high-grade metal microelectronics with controllable surface morphology and 3D dimensions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Additive manufacturing, also known as 3D printing, is a proven technology that allows intricated metal structures to be freely formed. Conventional thermal 3D printing technology, such as fused filament fabrication [1], selective laser melting [2], and inkjet printing [3], has been widely used in the microfabrication of precision metal electronics such as integrated circuits, fluidic devices, and flexible electronic components [4,5,6,7]. On the other hand, the products manufactured by these thermal processes are often subject to surface distortion and defect generation upon high-temperature treatment in the nitrogen atmosphere [8,9,10] which to some extent cannot meet their application requirement of high electrical conductivity [11], light response [12], thermal stability [13], and catalytic activity [14]. As an emerging additive manufacturing technology, electrochemical 3D printing exhibits compelling advantages in fabricating defect-free metal microstructures at ambient temperature by layered reducing metal atoms on the cathode surface.
Meniscus-confined electrodeposition (MCED), one of the most popular electrochemical 3D printing technologies, has been used to print sturdy metal microstructures through dynamic meniscus confinement. Hu et al. first proposed the concept of electrolyte meniscus, a three-phase interface consisting of electrolyte, substrate, and air [15]. A low electrolyte concentration was maintained during the MCED process to prevent the clogging of a microscale nozzle. Wang et al. established a way of controlling the contact and withdrawal of nozzles relative to the substrate with pre-set threshold currents to avoid nozzle blockage and meniscus rupture [16], while Hengsteler et al. employed the same principle and nanoscale nozzles to determine the MCED’s limit printing resolution of 25 nm [17]. Despite the notably improved continuity and deposition positioning of MCED, the slow nozzle motion leads to a low deposition rate. Chen and co-workers illustrated the possibility of printing sizable structures with a larger 0.4-mm-diameter nozzle to form a meniscus containing 1 M concentration of copper sulphate (CuSO4) and printed Cu wires with a Vickers hardness up to 228 MPa [18]. Zhang et al. further demonstrated that by adjusting the meniscus electrochemical gradients, a combined gradient of potential and metal ion concentration could print nanotwinned Cu structures with an extreme hardness of up to 2.4 GPa [19]. Nevertheless, the electrolyte meniscus is susceptible to humidity [19], temperature [20], evaporative ion convection [21], and nozzle movement status [16, 17], resulting in undesirable morphologies, low printing efficiency, and irreproducible microstructures, thus restraining MCED from the fabrication of microelectronic components with smooth surfaces.
On the other hand, jet electrochemical deposition (JECD) enables more efficient manufacturing of metal structures by injecting metal ions through an electrolyte jet onto the cathode surface, regardless of the rigorous liquid–gas-solid interfaces [22]. It was reported by Wang et al. that the electropolishing-assisted JECD produced a 2D ultra-flat Cu coating with surface roughness (SR) down to 13.5 nm [23]. Kim and co-workers achieved selective metallization of Cu patterns with SR of 2 μm even on the non-conductive substrates [24]. These achievements suggest promising prospects of JECD for direct printing smooth Cu microelectrodes [25], 3D-stacked catalysts [26], and multifunctional sensors on flexible polymer [27]. Furthermore, Rajput et al. revealed that the electrolyte jet speed, Cu2+ concentration, potential, and inter-electrode gap affect the deposition rate and deposition localization of JECD [28]. An increase in current density with a Gaussian distribution leads to a higher mass transfer rate but induces a wider diffusion area [29]. Although the deposition accuracy was improved by using compressed air films and dichloromethane to confine the electrolyte jet [30, 31], only a relatively lower deposition rate was maintained. Usually, Cu grains refine with increased current density in direct current ECD [32], but the sizes of grains produced by air–liquid constrained JECD become larger instead, leading to a further rise in SR.
Furthermore, it was demonstrated that the circulating electrolyte flow could achieve an effective grain refinement and uniform plating of Cu evenly through the depositional trenches and pores [33]. For instance, the electrolyte circulation system designed by Kim et al. can simultaneously inject and recover electrolytes up to 80 kPa through the electrode nozzle and two suction channels located on both sides [24]. However, the maximum jet pressure is entirely dependent on the threshold value of the pressure regulator. The 4 m. s−1 of electrolyte jet speed reported by Ning and co-workers is the fastest to date and revealed that the highest current efficiency and smallest diffusion area occur when the jet speed reaches above 3 m. s−1 [34]. In the present work, a cyclic high-speed electrolyte jet (HSEJ) 3D printer built on a portable 3-axis platform was first developed. It can print ultrasmooth and robust microelectrodes with waste Cu cable based on the circulating JECD principle. According to the characterization results by field emission electron microscopy (FESEM), the effects of current density on the Cu surface nucleation were investigated. SR measured by atomic force microscopy (AFM) and microhardness tested by a Vickers hardness tester were employed to analyse the microelectrode surface quality. The correlation between the cathode current efficiency and deposition localization was further studied. The findings present in this work open a new avenue for the low-cost manufacturing of precision microelectronics with multifunction and sophisticated geometries.
Experimental
Materials and chemicals
A 1-mm-diameter and 15-mm-long Cu wire extracted from abandoned commercial cables was used as the anode and polished in a flat end for each experiment. One piece of 5 cm2 Cu foil tape (Eco-Fused) with 0.15-mm thickness adhered to the printing platform as the cathode. A dilute hydrochloric acid solution was used to remove the oxide layer of the anode and cathode. Then, they were rinsed with ethanol several times and dried at 70 °C for 30 min. The saturated CuSO4 electrolyte with a concentration of 15.5% w/w was prepared using copper sulphate pentahydrate (CuSO4·5H2O, > 98% purity, Sigma-Aldrich). The mixed electrolytes were prepared by adding a nominal amount of potassium nitrate (KNO3, > 99% purity, Sigma-Aldrich) into the saturated CuSO4 to obtain 2% w/w, 4% w/w, and 6% w/w concentrations of KNO3, respectively. The electrolyte conductivity was measured by a precision bridge (LCR 400), and the electrolyte pH was tested by a benchtop meter (pH 700, OAKTON).
Electrochemical measurement
The chronoamperometry experiments were carried out using a potentiostat (SP-300, BioLogic) at a scanning interval of 0.5 s to obtain the cathode current over time at three potentials of 2.5, 5 and 7.5 V. The reference electrode and counter electrode from the potentiostat were connected in series with a Cu wire anode and combined with the working electrode that connected to the Cu foil cathode to establish a two-electrode system. The cathode reaction during the printing process is shown below:
According to Faraday’s law of electrolysis, the theoretical deposition mass (wt) is calculated based on Eq. (1), where M is the molar mass of Cu, I is cathode current, T is printing time, N is displaceable electrons per Cu atom, and F is Faraday’s constant. The cathode current efficiency (\({\upeta }_{c}\)) is calculated by Eq. (2), according to actual deposition mass (wa) under each printing condition.
Characterization and mechanical test
A FESEM (JEOL-JSM 7100F) was used to obtain the surface morphological images of the microelectrodes at 15 kV. The printed microelectrodes were placed in ethanol under sonication and subsequently dried at 60 °C for 30 min. Then, the particle distribution calculation software (Nano Measurer 1.2) was employed to assess the Cu nuclei size and nuclei population density based on the SEM images. Roughness was measured by atomic force microscopy (AFM, Bruker Innova) with a threshold height of 0.8 nm on the microelectrode surface. The microelectrode was embrittled and broken from the centre in liquid nitrogen. The deposition width and thickness were measured based on the top view and cross-sectional view taken by FESEM. The maximum surface microhardness was tested by a Vickers hardness tester (DuraScan 10/20 G5) at a Vickers pyramid number of 0.2 according to the mean diagonal length of the diamond-shaped pit indented on the microelectrode surface.
Results and discussion
High-speed electrolyte jet 3D printing
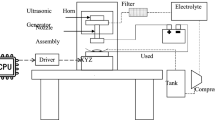
As shown in Fig. 1, the desktop electrochemical 3D printer was adapted from a fused filament fabrication single-head printer (Felix 3.0). The major improvement was replacing the original extruder with an electrochemical nozzle that was assembled by a 1-ml syringe, a pipette tip, and a gas-tight syringe holder that was fixed to the X-axis stepper motor. As shown in Table 1, the nozzle tip possesses an outlet diameter of 0.5 mm and was positioned 0.5 mm from the substrate to prevent the nozzle from clogging. A 50-ml glass beaker with a central printing platform was used as the container. Due to the high sensitivity of metals to electrochemical reactions, both the syringe and pipette tip were made of polypropylene. The syringe holder and the printing platform were 3D printed with acrylonitrile butadiene styrene. Furthermore, an adjustable buck-printed circuit board was used as a direct current (DC) power supply to ensure a constant deposition current for each potential setting. The electrolyte circulation system was established by connecting the electrochemical nozzle to a homemade peristaltic pump with an 80 ml. min−1 circulating rate as recorded in Table 1. The 2,500 printing passes were set bi-directionally along a 5-mm length by an in-house developed computer numerical control programming, enabling a fully automatic and continuous microelectrode fabrication in ambient conditions.
As the magnified view displayed in Fig. 1 (right), a top-embedded waste Cu cable was used as the anode to play the role of a cheap ion source. The Cu2+ ions released by the oxidative dissolution continuously replenish the ion depletion in the CuSO4 electrolyte to maintain a saturated state. Like the filament, the waste cable becomes the sole consumable for a prolonged and stable printing process, offering a more sustainable pathway than using inert anodes requires periodic electrolyte renewal [35, 36]. Most research priorities of JECD are independent and mainly focus on process parameter optimization, mechanical property enhancement, and deposition localization improvement [28, 31], respectively. This work is chiefly on recycling waste cables into metal products with certain thicknesses, where achieving high surface quality under an adjustable deposition localization becomes the primary challenge. The nozzle parameters listed in Table 1 were fixed to control the mechanical variables for intensively studying the effects of KNO3 concentration and applied potential on Cu nucleation behaviour. The electrolyte was delivered by a high jet speed of 6.8 m/s to the negatively charged Cu foil substrate for Cu deposition with desirable current efficiency. To diminish the negative impacts of vigorous nozzle movement on printing continuity [37], the Cu microelectrodes were formed at a nozzle scanning velocity of 6 mm. s−1, the minimum allowed by the commercial platform.
Chronoamperometry
The proper addition of inert KNO3 instead of sulphuric acid as a pH equalizer and conductivity promoter is a prerequisite for fabricating ultrasmooth Cu surfaces. It is understood that K+ ion additives are commonly used in conventional ECD processes to complex with Cu2+ ions to reduce the effect of hydrogen evolution on Cu nucleation behaviour [38]. Although it has been revealed that the addition of moderate amounts of strong acids into the aqueous electrolyte can significantly promote the metal deposition rate [39], the irregular hydrogen bubble generation, low electrolyte pH (< 1), and the overlapping of the ion diffusion fields were proved to induce the surface stacking and the dendrite growth which may increase the SR of the metal. Upon the addition of KNO3 into the CuSO4 (sat.) electrolyte, the electrolyte conductivity increases rapidly from 5 to 34.2 S/m with the increased concentration of KNO3 as shown in Table 2, while the cathode current exhibits a gradual growth at a constant potential of 5 V. It implies that adding inert KNO3 can finely tune the cathode current at a constant pH value of the CuSO4 (sat.) electrolyte. Zhang et al. illustrated that the electrochemical gradients existing at a relative humidity above 65% are not conducive to transferring large amounts of Cu2+ ions onto the cathode surface [19]. Although the electrochemical gradient has a negligible influence on the JECD, the printing process was carried out at 23 °C and relative humidity of 60% to minimize the impact from varying Cu2+ concentration gradient induced by water evaporation.
It can be seen from Fig. 2 that the variations of current density over time present relatively flat trends in the absence and presence of 2% w/w KNO3, which may be attributed to a negligible change in electrolyte conductivity. As the KNO3 concentration increases to 4% w/w, the electrolyte conductivity increases from 8 to 26.9 S/m, triggering three instantaneous peaks. It can be explained that the dramatically increased electrolyte conductivity may lead to a transient excess of deposition energy inducing secondary nucleation to form the nanopyramid at the average current density of 0.29 mA. cm−2. At further addition of the KNO3 up to 6% w/w, the instantaneous peak disappears, and a more drastic fluctuation of current density occurs, which can explain the appearance of the dense and nonporous nucleation morphology at 0.32 mA. cm−2. It seems that the variations in current density in terms of both average values and consequent fluctuation patterns can affect the primary and secondary nucleation on the Cu surface. It is also noted that the potential exerts a significant influence on the current density, thus impacting the surface nucleation. At a low current density of 0.15 mA. cm−2 and a high current density of 0.49 mA. cm−2, the instantaneous peaks occur when KNO3 concentration remains constant at 6% w/w, leading to the reformation of Cu crystals.
The nucleation morphologies at the average current densities of 0.21, 0.25 and 0.32 mA. cm−2 show that the primary nucleation becomes more compact as the current density’s fluctuation intensifies possibly due to the increased transfer flux of Cu2+ ions to the cathode surface, resulting in a higher frequency of nucleation. Nonetheless, as the average current densities rise from 0.15 mA. cm−2 to 0.29 and 0.49 mA. cm−2, respectively, the size of Cu pyramidal crystals is inversely proportional to the instantaneous peak amplitude, demonstrating that the peak current density may affect the secondary nucleation on the Cu surface. It also can be observed that the instantaneous peak appears once at the highest average current density and then climbs to 3 and 9 times, respectively, as the average current density drops to 0.29 mA. cm−2 and 0.15 mA. cm−2. It is illustrated that the average current density, directly proportional to the electric field strength based on Ohm’s law, may determine the critical deposition energy for each printing parameter, resulting in an apparent negative correlation between the instantaneous peak amplitude and its occurrence frequency.
Analysis of Cu nucleation morphology and nuclei size distribution
Figure 3 displays the SEM images of the Cu microelectrode produced at different printing conditions. As shown in Fig. 3a, the microelectrode surface is covered by large Cu grains and pyramidal crystals in mean sizes of 4.06 µm and 2.45 µm, respectively, at a low current density of 0. 15 mA. cm−2. The wide distribution range of grain size from 1 to 9 µm may be attributed to the formation of microcracks and grooves on the microelectrode surface as seen in the image. Figure 3b depicts the grain refinement to 3.13 µm when the average current density increases to 0.21 mA. cm−2. Similar to the nuclei size distribution at 0.15 mA. cm−2, the grains with sizes between 2 to 3 µm still dominate the distribution population. The Cu pyramids generated from secondary transient nucleation disappear, accompanied by the surface defective pattern transforming from cracking to pore-like. Figure 3c presents a tomographic distribution of the Cu grain as the average current density further enhances to 0.25 mA. cm−2. There is a sharp reduction in mean grain size to 1.44 µm, mainly due to the inner porous defects migrating to the surface of the microelectrode as illustrated in schematic (b-c). Cross-nucleation [40], defined as the process of daughter nuclei derived from polycrystalline parents, may occur at a higher current density of 0.29 mA. cm−2, where most grains are nucleated between 1—1.2 µm with a narrow size distribution. As shown in Fig. 3d and schematic (c-d), Cu nanopyramids with a mean size of 0. 69 µm were the daughter crystals generated adjacent to their parent grains and uniformly grew as part of the primary nucleation layer.
SEM surface morphologies, schematic of pore-like defects transforming to the defect-free surface, and corresponding nuclei size distribution (d represents mean nuclei size) of Cu microelectrodes produced at different printing conditions and relative average current densities of a 0.15, b 0.21, c 0.25, d 0.29, e 0.32, and f 0.49 mA. cm−2.
Figures 3e and schematic (d-e) depict the formation of the defect-free surface due to the enhanced lateral Cu nucleation at a higher concentration of K+ ions and an increased current density of 0.32 mA. cm−2. The mean grain size dramatically reduces by 0.77 µm and evenly self-assembles a dense nucleation layer, indicating the termination of cross-nucleation. As shown in Fig. 3f, Cu nanograins in size of 0.29 µm were formed with more densely distributed nanopyramids in size of 0.48 µm at the highest current density of 0.49 mA cm−2. Despite the smaller nucleation of grains and pyramids, the uneven surface consisting of localized grain aggregation may be attributed to the irregular reduction of Cu2+ ions caused by exceeding the limiting current density for successive nucleation [41]. The Cu nucleation mechanism under HSEJ is quite consistent with the conventional ECD. For example, Pesic and co-workers illustrated that the high Cu2+ concentration was conducive to generating larger Cu nuclei [42], which aligns with the Cu grains formed in size above 1 µm as presented in Fig. 3a–d due to the saturated electrolyte jet. When electroplating Cu coatings on aluminium substrates, Augustin et al. found that the Cu grain size decreased to the nanoscale with rising current density up to 100 mA. cm−2 [43], corresponding to the smaller nuclei size distribution presented in Fig. 3e, f. Nevertheless, HSEJ 3D printing refines the grains and crystals at a much lower current density by repeatedly and uniformly laying Cu nuclei.
Effects of nucleation behaviour on surface roughness and microhardness
As shown in Entry 1 Table 3, the microelectrode surface covered by a Cu grain size of 4.06 µm and a crystal size of 2.45 µm possesses a surface average roughness (Ra) of 1.2 nm. Natter and co-workers demonstrated that higher potentials promote nucleation rates and current densities, corresponding to slower grain formation [44]. The low potential may reduce the rates of twice nucleation, leading to the rapid aggregation of adjacent Cu atoms to form large grains and crystals, while a high K+ concentration improves the homogeneity of nucleation, resulting in a lower Ra. On the other hand, the microelectrode surface that solely consists of smaller Cu grains in sizes of 3.13 µm and 1.44 µm in Entry 2 and 3 have a dramatic rise in Ra up to 27.4 nm and 27.8 nm, respectively. The difference in Ra becomes the most pronounced in Entry 3 and 4 due to the disappearance of surface defects (Fig. 3b, c). In contrast, there are no apparent variations of Ra occur in Entry 4 to 5 when progressing from cross-nucleation (Fig. 3d) to dense nucleation (Fig. 3e). Although the grain size of 0.29 µm obtained in Entry 6 is the lowest, the more serious grain aggregation and denser crystal distribution due to the faster nucleation at more extensive potential, significantly climb the Ra to 24.8 nm. Accordingly, the uniform and slow grain growth provided by adding 6% w/w KNO3 at a medium potential contributes to the minimum Ra, rather than the grain refinement induced by higher current density.
In addition, the variation of Cu grain size reflects the uniformity of grain boundary distribution, essentially affecting the mechanical behaviour of Cu microelectrodes. During the thermal 3D printing processes [45, 46], the mechanical strength of metal microstructures usually increases with grain refinement known as the Hall–Petch effect [47]. It was reported by Ghassemali and co-workers that the softening of the metal material occurred in the case of recrystallization caused by annealing treatments even when the grain size was reduced to submicron [48]. Since the conventional ECD is carried out at ambient temperatures, several studies have demonstrated the Hall–Petch relationship between the microhardness and deposited grain size [32, 43, 49]. Nonetheless, the HSEJ in the present work caused the Cu nucleus to partially detach from the primary nucleation surface leading to a continuous or separate secondary nucleation. It is introduced in Fig. 4 that the microhardness of the Cu microelectrodes increases dramatically from 1.6 to 3.3 GPa as the grain size shrinks from 3.13 to 0.34 µm with a decline in grain size variation. Although the Cu pyramids with a relatively higher population density of \(2.1\times {10}^{6}\) cm−2 existed in Entry 4 Table 3, the Cu grains experienced a negligible size variation from the extrusion dislocations due to the overlap of the primary and second nucleation (Fig. 3d), thereby improving the surface microhardness of the Cu microelectrode.
Alternatively, the presence of Cu crystals at the average current density of 0.49 mA. cm−2 (Fig. 3f) prompts a significant difference among the Cu grain sizes represented by a variation of 41.4%, resulting in mechanical behaviour that may defy the Hall–Petch effect. Conrad et al. demonstrated that the reverse Hall–Petch effect is induced when the grain size is smaller than the critical value of 10 nm, causing the disappearance of grain boundary dislocations [50], which to some extent explains the phenomenon of decreased microhardness with the grain refinement from 0.34 to 0.29 μm, as displayed in Fig. 4. In contrast to the current findings of Hall–Petch effect in the situation of non-secondary nucleation [51], the high coverage of Cu nanopyramids leads to the increased grain size variation from 23.5 to 41.4% may express the earlier grain boundary sliding [52], resulting in the microelectrodes softening even at the grain size of hundred nanometres.
It can be seen from Table 3 and Fig. 4 that a clear inverse correlation exists between Ra and microhardness at an identical potential of 5 V in Entry 2–5, which is probably due to the more intensive nucleation of Cu2+ at a higher current density. The grain refinement driven by the increased potential at the same KNO3 concentration in Entry 1, 5, and 6 plays a dominant role in determining the microhardness. In this work, the best-printed Cu microelectrode composed of nanograins in size of 0.34 µm possesses a minimum Ra of 1.1 nm and a microhardness up to 3.3 GPa, illustrating the feasibility in the mass production of high-grade microelectrode. Although the denser population of Cu pyramids from Entry 1, 4, to 6 may result in a rougher surface and the possible reverse Hall–Petch effect, they have been evidenced the excellent photoelectric properties [53] and were widely used as highly active cathode materials [54,55,56], exhibiting versatility of HSEJ 3D printed metal products.
Influence of printing conditions on the Cu deposition mass
As introduced in Fig. 5, the increasing KNO3 concentration has less impact on the deposition mass of the Cu microelectrode than that offered by changing potentials. At 5 V of applied potential, the deposition mass slightly climbs from 1.3 to 1.6 mg at the initial addition of 2% w/w KNO3 and then remains constant. The presence of K+ in the CuSO4 electrolyte was reported to provide a more negative reduction potential for transient Cu nucleation at a concentration of Cu2+ above 10 mM [38], explaining the increase in deposition mass after the same printing passes compared to that without the KNO3 additive. When enhancing potentials from 2.5 to 7.5 V at 6% w/w KNO3, the deposition mass dramatically jumps from 1 to 1.6 and 2.2 mg, illustrating the dominant effect of applied potential on regulating the Cu nucleation rate. It was evidenced that the larger potential had a promotional effect on the increase in nuclei population density [42], corresponding to the huge difference in size and population density from twice nucleation (Entry 1 and 6 Table 3) at 2.5 and 7.5 V. In comparison, the modifications in Cu nucleation parameters (Entry 3–5 Table 3) with increasing KNO3 concentrations at a constant potential of 5 V become less apparent. Therefore, the denser and smaller Cu nuclei growing per unit area at larger potential mainly contributes to the higher deposition mass.
Influence of printing conditions on 3D dimensions and deposition localization
The ultrasmooth Cu microelectrode schematically displayed in Fig. 6a exhibits a shiny surface with the outer edges appearing dark brown. A micro-illustration of the difference in total deposition width and diffusion width is shown in Fig. 6b. The double diffusion layers were formed along the nozzle scanning direction, which may be attributed to the vertical and lateral combined diffusive growth from the jet disturbance at the early impingement [57]. Figure 6f introduces the correlation between deposition localization and cathode current efficiency, where an overall declining trend of the current efficiency appears with rising average current density. The only point against this trend is that the current efficiency increases accompanied by a 4% narrower percentage of diffusion width to the total width at 0.25 mA. cm−2 compared to that achieved at 0.21 mA. cm−2. It was also revealed that the less diffusive ion migration from the stable electrolyte jet provided regularly arranged nucleation sites thereby inhibiting the diffusion deposition [57]. The abnormal jump in current efficiency may be due to the addition of 2% w/w KNO3 which initially creates more lateral nucleation sites and obstructs the Cu diffusion. On the other hand, the current efficiency is slightly higher with 6% w/w KNO3 addition at 0.15 mA. cm−2, suggesting that more Cu2+ ions were reduced before exceeding the limiting current density [58]. Although the total width expands with a higher average current density, the current efficiency is negatively correlated with the diffusion percentage, reflecting a continuous lowering in deposition localization at above 0.25 mA.cm−2.
a Picture of the ultrasmooth Cu microelectrode printed at 6% w/w KNO3 and an applied potential of 5 V. SEM images of b the top view and c the cross-sectional view of the ultrasmooth Cu microelectrode. Cross-sectional microstructure evolution from the d centre to the e double edges before the diffusion layer. f The relationships among the current efficiency, deposition width, and diffusion percentage with the increasing average current density. g Effects of KNO3 concentration and applied potential on deposition thickness.
Figure 6c–e depicts the cross-sectional morphology of the ultrasmooth microelectrode and the microstructure transformation from the deposition centre to both edges. As shown in Fig. 6c, the cross section of the microelectrode becomes prismatic with visible walls, due to the shedding of the loose diffusion layer after cryogenic embrittlement. The microstructure printed directly underneath the nozzle (Fig. 6d) becomes more compact and smoother than that along the edges (Fig. 6e), which is because of the much smaller potential distribution with the farther distance from the electrolyte jet centre [28]. Also, an inverse relationship between KNO3 concentration and deposition thickness is shown in Fig. 6g, while the influence of modifying potential is negligible. At a constant potential at 5 V, the microelectrodes’ thickness decreases from 105 to 62 µm with rising KNO3 concentration. Majidi et al. proved that the accumulation of K+ ions in both the body layer and the diffusion layer promoted regular Cu reduction [38]. They also mentioned a combined interference of diffusive ions and their mass transfer rate on the multi-dimensional Cu nucleation at an electrolyte pH between 2 and 3 [38]. Corresponding to the situations at pH of 2.8–2.9 described in Fig. 6f, g, the deposition width of the Cu microelectrodes exhibits an obvious negative correlation with thickness at an unchanged deposition mass (Fig. 5). On the other hand, applying higher potential at the same KNO3 concentration individually affects the deposition width and mass. These findings emphasize the dominant role of KNO3 and the applied potential for ion diffusion and ion mass transfer, respectively. The high concentration of KNO3 transferring the residual and diffusive Cu2+ ions to the double edges facilitates the smoother surface of Cu microelectrodes.
According to the deposition thickness obtained at the 6% w/w KNO3 and 5 V after a 1.16 h printing, the ultrasmooth microelectrode possesses a much faster deposition rate of 53.4 µm/h which is 3.5 times that obtained by MCED at 4 V [18]. On the other hand, there is a certain gap between the Cu deposition rate of the HSEJ 3D printer and that reported in other JECD processes. For instance, Chen et al. achieved a deposition rate of up to 0.3 µm/s when fabricating the tower microstructures [59]. Wang and his co-workers deposited micropillar at a higher deposition rate of 20 µm/s [60]. This is attributed to the different deposition directions, while the maximum deposition rate of 2.5 µm/s from nozzle scanning is still slower [61]. For scanning JECD, Jiang and his colleagues have proved the dominant effect of higher current density on facilitating the deposition rate accompanied by lower SR and enhanced surface microhardness rather than nozzle scanning velocity [62]. However, a limiting current density appears to define the threshold of the stable deposition. It was further evidenced in Duan et al.’s work that the deposition thickness first raised and then declined as exceeding the limiting current density, where the variation of metal grain size was reversed [63]. In comparison, our results illustrate that the grain size (Fig. 3) is directly proportional to deposition thickness (Fig. 6g) when adding more KNO3 at 5 V, ensuring the formation of smooth and robust microelectrode surfaces below the limiting current density. Although the higher Cu2+ ion concentration and larger applied potential can promote the deposition rate [62], the microelectrodes printed at a more extensive potential of 7.5 V obtain a similar thickness to that at 2.5 V. The presence of uneven surface (Fig. 3f) with higher Ra (Table 3) suggests that diffusive and discontinuous Cu nucleation begins to dominate the 3D printing process at 7.5 V, which implies that the current density of 0.49 mA. cm−2 may exceed the limiting value.
Conclusions
In this study, the desktop HSEJ 3D printer was firstly reported to recycle waste Cu cables into microelectrodes with controllable surface morphology and 3D dimensions, offering a cost-effective way for facile printing microelectronic components. The cathode current density obtained by the chronoamperometry correlates well with the nucleation behaviour of Cu microelectrodes. The average current density is directly proportional to the deposition width and the grain size, and then the instantaneous peak current density is negatively correlated with the pyramidal crystals’ size and population density. Also, a high current density induces grain refinement, and its drastic fluctuation enables the formation of dense nucleation surfaces. Due to the limited deposition energy, the instantaneous peak of current density is inversely proportional to its occurrence frequency for each printing condition, resulting in the different secondary nucleus sizes and impacting the Ra of microelectrodes. The pyramidal Cu crystals that appeared on the microelectrode surface exhibit the promising prospects that HSEJ 3D printing can produce multifunctional microelectrodes. Besides, the larger potential promotes denser nucleation thus positively correlating with deposition mass, while higher KNO3 concentration inhibits the diffusive nucleation on the microelectrode surface thereby inversely proportional to the deposition thickness. Despite the higher current density creating a wider deposition, the diffusion percentage under free electrolyte flow is entirely determined by the current efficiency, enabling a precise adjustment of the deposition localization.
Data availability
Data supporting the results of this study are available upon request from the corresponding author.
References
Flowers PF, Reyes C, Ye S, Kim MJ, Wiley BJ (2017) 3D printing electronic components and circuits with conductive thermoplastic filament. Addit Manuf 18:156–163
Ikeshoji T-T, Nakamura K, Yonehara M, Imai K, Kyogoku H (2018) Selective laser melting of pure copper. JOM 70:396–400
Kang JS, Kim HS, Ryu J, Thomas Hahn H, Jang S, Joung JW (2010) Inkjet printed electronics using copper nanoparticle ink. J Mater Sci Mater Electron 21:1213–1220
Wu S-Y, Yang C, Hsu W, Lin L (2015) 3D-printed microelectronics for integrated circuitry and passive wireless sensors. Microsyst Nanoeng 1:15013
Lee KG, Park KJ, Seok S, Shin S, Kim DH, Park JY, Heo YS, Lee SJ, Lee TJ (2014) 3D printed modules for integrated microfluidic devices. RSC Adv 4:32876–32880
Zhou X, Guo W, Fu J, Zhu Y, Huang Y, Peng P (2019) Laser writing of Cu/CuxO integrated structure on flexible substrate for humidity sensing. Appl Surf Sci 494:684–690
DebRoy T, Wei HL, Zuback JS, Mukherjee T, Elmer JW, Milewski JO, Beese AM, Wilson-Heid A, De A, Zhang W (2018) Additive manufacturing of metallic components—process, structure and properties. Prog Mater Sci 92:112–224
Tsai C-Y, Chang W-C, Chen G-L, Chung C-H, Liang J-X, Ma W-Y, Yang T-N (2015) A study of the preparation and properties of antioxidative copper inks with high electrical conductivity. Nanoscale Res Lett 10:357
Goto I, Kurosawa K, Matsuki T (2022) Effect of 3D-printed sand molds on the soundness of pure copper castings in the vicinity of as-cast surfaces. J Manuf Process 77:329–338
German RM (2016) Sintering trajectories: description on how density, surface area, and grain size change. JOM 68:878–884
Jun HY, Lee EJ, Ryu SO (2020) Synthesis and characterization of copper ink and direct printing of copper patterns by inkjet printing for electronic devices. Curr Appl Phys 20:853–861
Salea A, Prathumwan R, Junpha J, Subannajui K (2017) Metal oxide semiconductor 3D printing: preparation of copper(ii) oxide by fused deposition modelling for multi-functional semiconducting applications. J Mater Chem C 5:4614–4620
Yu J, Sun X, Tong X, Zhang J, Li J, Li S, Liu Y, Tsubaki N, Abe T, Sun J (2021) Ultra-high thermal stability of sputtering reconstructed Cu-based catalysts. Nat Commun 12:7209
Amadine O, Essamlali Y, Fihri A, Larzek M, Zahouily M (2017) Effect of calcination temperature on the structure and catalytic performance of copper–ceria mixed oxide catalysts in phenol hydroxylation. RSC Adv 7:12586–12597
Hu J, Yu M-F (2010) Meniscus-confined three-dimensional electrodeposition for direct writing of wire bonds. Science 329:313–316
Wang Y, Chen Y-L, Wang Y, Ju B-F (2020) A method for micropipette-based meniscus-confined electrodeposition of microstructures without nozzle clogging. Nanomanufactur Metrol 3:77–82
Hengsteler J, Mandal B, van Nisselroy C, Lau GPS, Schlotter T, Zambelli T, Momotenko D (2021) Bringing electrochemical three-dimensional printing to the nanoscale. Nano Lett 21:9093–9101
Chen X, Liu X, Childs P, Brandon N, Wu B (2017) A low cost desktop electrochemical metal 3D printer. Adv Mater Technol 2:1700148
Zhang X, Yuan L, Lei Y, Zhang Y, Li Y, Nie W, Gao Q, Li Z, Sun A, Liu F, Cheng Y, Xu G, Guo J (2021) Electrochemical gradients driven 3D printing of nano-twinned copper structures by direct current dynamic meniscus confined electrodeposition. Appl Mater Today 24:101138
Xu J, Xu Z, Ren W, Zou Z, Wang X (2022) Simulation and experimental study on metal microstructure of meniscus-confined electrodeposition. Adv Eng Mater 24(12):2200654
Seol SK, Kim D, Lee S, Kim JH, Chang WS, Kim JT (2015) Electrodeposition-based 3D printing of metallic microarchitectures with controlled internal structures. Small 11:3896–3902
Kunieda M, Katoh R, Mori Y (1998) Rapid prototyping by selective electrodeposition using electrolyte jet. CIRP Ann 47:161–164
Wang K, Yan Y, Zhou P, Zhang C, Kang R, Guo D (2020) Preparation of flat and smooth copper surface by jet electrochemical machining and electrochemical polishing. J Electrochem Soc 167:163501
Kim H, Kim JG, Park JW, Chu CN (2018) Selective copper metallization of nonconductive materials using jet-circulating electrodeposition. Precis Eng 51:153–159
Lian L, Wang H, Dong D, He G (2018) Highly robust and ultrasmooth copper nanowire electrode by one-step coating for organic light-emitting diodes. J Mater Chem C 6:9158–9165
Kim YJ, Lim A, Kim JM, Lim D, Chae KH, Cho EN, Han HJ, Jeon KU, Kim M, Lee GH, Lee GR, Ahn HS, Park HS, Kim H, Kim JY, Jung YS (2020) Highly efficient oxygen evolution reaction via facile bubble transport realized by three-dimensionally stack-printed catalysts. Nat Commun 11:4921
Dong Y, Min X, Kim WS (2018) A 3-D-printed integrated PCB-based electrochemical sensor system. IEEE Sens J 18:2959–2966
Rajput MS, Pandey PM, Jha S (2015) Modelling of high speed selective jet electrodeposition process. J Manuf Process 17:98–107
Fan H, Zhao YP, Wang SK (2016) Technical study of jet electrodeposition in manufacture of metal parts. Key Eng Mater 667:259–264
Xinchao L, Pingmei M, Xinmin Z, Wei W, Yanhua Z, Ge Q, Xingshuai Z, Shen N (2020) Compressed air-film encircling jet electrodeposition with high deposition accuracy. J Electrochem Soc 167:102502
Chen X, Chen J, Saxena KK, Zhu J, Gu X, Guo Z (2022) Localization of jet electrochemical additive manufacturing with a liquid confinement technique. J Manuf Process 81:48–64
Ibañez A, Fatás E (2005) Mechanical and structural properties of electrodeposited copper and their relation with the electrodeposition parameters. Surf Coat Technol 191:7–16
Lee P-T, Chang C-H, Lee C-Y, Wu Y-S, Yang C-H, Ho C-E (2021) High-speed electrodeposition for Cu pillar fabrication and Cu pillar adhesion to an Ajinomoto build-up film (ABF). Mater Des 206:109830
Ning D, Yang C, Wu H (2019) Ultrafast Cu2+ recovery from waste water by jet electrodeposition. Sep Purif Technol 220:217–221
Xiao Y, Ming P, Zhang X, Hou Y, Du L, Li S, Zhang Y, Song J (2022) Ultrahigh strength ultrapure nanostructured nickel metal prepared via ultrafine anode scanning electrodeposition. Mater Des 213:110339
Zhang Y, Ming P, Xue B, Liu H, Yang X, Li L, Niu S, Yan L, Zheng X, Qin G (2023) Facilely fabricating large-area robust heterogeneous wettability surface by mask-patterned ultrafine anode scanning electrodeposition for efficient water collection. Surf Interfaces 41:103247
Lei Y, Zhang X, Xu D, Yu M, Yi Z, Li Z, Sun A, Xu G, Cui P, Guo J (2018) Dynamic “Scanning-Mode” meniscus confined electrodepositing and micropatterning of individually addressable ultraconductive copper line arrays. J Phys Chem Lett 9:2380–2387
Majidi MR, Asadpour-Zeynali K, Hafezi B (2009) Reaction and nucleation mechanisms of copper electrodeposition on disposable pencil graphite electrode. Electrochim Acta 54:1119–1126
Rosa-Ortiz SM, Khorramshahi F, Takshi A (2019) Study the impact of CuSO4 and H2SO4 concentrations on lateral growth of hydrogen evolution assisted copper electroplating. J Appl Electrochem 49:1203–1210
Looijmans SFSP, Cavallo D, Yu L, Peters GWM (2018) Cross-nucleation between polymorphs: quantitative modeling of kinetics and morphology. Cryst Growth Des 18:3921–3926
Ibl NI, Schadegg K (1967) Surface roughness effects in the electrodeposition of copper in the limiting current range. J Electrochem Soc 114:54
Grujicic D, Pesic B (2002) Electrodeposition of copper: the nucleation mechanisms. Electrochim Acta 47:2901–2912
Augustin A, Huilgol P, Udupa KR, Bhat U (2016) Effect of current density during electrodeposition on microstructure and hardness of textured Cu coating in the application of antimicrobial Al touch surface. J Mech Behav Biomed Mater 63:352–360
Natter H, Hempelmann R (1996) Nanocrystalline copper by pulsed electrodeposition: the effects of organic additives, bath temperature, and pH. J Phys Chem 100:19525–19532
Todaro CJ, Easton MA, Qiu D, Zhang D, Bermingham MJ, Lui EW, Brandt M, StJohn DH, Qian M (2020) Grain structure control during metal 3D printing by high-intensity ultrasound. Nat Commun 11:142
Wang Y, Wang Y-T, Li R-D, Niu P-D, Wang M-B, Yuan T-C, Li K (2021) Hall-Petch relationship in selective laser melting additively manufactured metals: using grain or cell size? J Cent South Univ 28:1043–1057
Naik SN, Walley SM (2020) The Hall-Petch and inverse Hall-Petch relations and the hardness of nanocrystalline metals. J Mater Sci 55:2661–2681
Ghassemali E, Tan M-J, Wah CB, Lim SCV, Jarfors AEW (2015) Effect of cold-work on the Hall-Petch breakdown in copper based micro-components. Mech Mater 80:124–135
Hakamada M, Nakamoto Y, Matsumoto H, Iwasaki H, Chen Y, Kusuda H, Mabuchi M (2007) Relationship between hardness and grain size in electrodeposited copper films. Mater Sci Eng A 457:120–126
Conrad H, Narayan J (2000) On the grain size softening in nanocrystalline materials. Scripta Mater 42:1025–1030
Carlton CE, Ferreira PJ (2007) What is behind the inverse Hall-Petch effect in nanocrystalline materials? Acta Mater 55:3749–3756
Schuh CA, Nieh TG (2003) Hardness and abrasion resistance of nanocrystalline nickel alloys near the hall-petch breakdown regime. MRS Online Proc Libr 740:18
Ko W-Y, Chen W-H, Tzeng S-D, Gwo S, Lin K-J (2006) Synthesis of pyramidal copper nanoparticles on gold substrate. Chem Mater 18:6097–6099
Chen L, Tang C, Jiao Y, Qiao S-Z (2021) Anomalous C−C coupling on under-coordinated Cu (111): a case study of Cu nanopyramids for CO2 reduction reaction by molecular modelling. Chemsuschem 14:671–678
Chen L, Tang C, Davey K, Zheng Y, Jiao Y, Qiao S-Z (2021) Spatial-confinement induced electroreduction of CO and CO2 to diols on densely-arrayed Cu nanopyramids. Chem Sci 12:8079–8087
Chen L, Tang C, Zheng Y, Skúlason E, Jiao Y (2022) C3 production from CO2 reduction by concerted *CO trimerization on a single-atom alloy catalyst. J Mater Chem A 10:5998–6006
Zhang F, Liu S, Wang F (2022) Nucleation and growth mechanism in the early stages of nickel coating in jet electrodeposition: a coarse-grained molecular simulation and experimental study. RSC Adv 12:11052–11059
Filzwieser A, Hein K, Mori G (2002) Current density limitation and diffusion boundary layer calculation using CFD method. JOM 54:28–31
Chen X, Gu X, Saxena KK, Arshad MH, Huang J, Reynaerts D (2023) Enhancing shape precision in jet electrochemical additive micro-manufacturing process through confined electrolyte. Precis Eng 84:69–80
Ye P, Niu Q, Wang F (2023) Effect of electrolyte composition and deposition voltage on the deposition rate of copper microcolumns jet electrodeposition. Mater Sci Eng B 298:116857
Bocking C, Cameron DB (1994) The use of high speed selective jet electrodeposition of gold for the plating of connectors. Trans IMF 72:33–40
Chen Y, Wen X, Li H, Zhu F, Fang C, Li Z, Zhou Z, Jiang W (2023) Effects of deposition current density, time and scanning velocity on scanning jet electrodeposition of Ni-Co alloy coating. J Manuf Process 101:458–468
Song Z, Zhang H, Fu X, Lin J, Shen M, Wang Q, Duan S (2020) Effect of current density on the performance of Ni–P–ZrO2–CeO2 composite coatings prepared by jet-electrodeposition. Coatings 10:616
Acknowledgements
We thank the Macquarie Analytical and Fabrication Facility for the use of microscopy units and mechanical tests. Financial support by the ARC Discovery Project (DP1901013720) and Macquarie University Research Excellence Scholarship for Mr Wending Gu is gratefully acknowledged.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
WG contributed to the conceptualization, methodology, and writing of the original draft. AM contributed to investigations. YJ contributed to the conceptualization, supervision, project administration, reviewing and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest to disclose.
Ethical approval
No experiments involving human participants and/or animals were carried out.
Additional information
Handling Editor: P. Nash.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gu, W., Marianov, A. & Jiang, Y. High-speed electrolyte jet 3D printing of ultrasmooth and robust Cu microelectrodes. J Mater Sci 59, 4605–4619 (2024). https://doi.org/10.1007/s10853-024-09514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09514-7