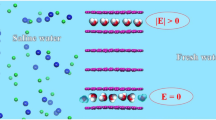
Abstract
Recent experiments have shown that using van der Waals assembly to stack graphene layers can facilitate extraordinary transport behavior of water and ions, paving the way for the design of new desalination devices utilizing graphene channels. However, the performance of pure graphene is inadequate due to the inherent trade-off between water permeability and ion selectivity. In this study, we conducted a series of molecular dynamics simulations and found that surface functionalization can significantly enhance the desalination efficiency of multilayer graphene channels. When maintaining a constant surface charge density, the fluxes of water and ions first increase slowly and then rapidly with the increase in channel height. Notably, the channel can always achieve an ion rejection rate of over 65% for cations and more than 95% for small channel heights. Conversely, for a given channel height, an increased surface charge density causes the fluxes of water and ions to decrease rapidly to zero and the ion rejection rate to rise quickly to 100%. Additionally, the translocation time, occupancy number, and electrostatic interaction with charged residues for water and ions are heavily influenced by the channel height and surface charge density. As a result, properly sized and surface-functionalized multilayer graphene channels have the potential to revolutionize desalination membrane design, allowing for high efficiency water filtration.
Graphical Abstract








Similar content being viewed by others
Data availability
There are no additional data. Detailed information can be provided by the corresponding author for request.
References
Alkhadra MA, Su X, Suss ME et al (2022) Electrochemical methods for water purification, ion separations, and energy conversion. Chem Rev 122:13547–13635
Aluru NR, Aydin F, Bazant MZ et al (2023) Fluids and electrolytes under confinement in single-digit nanopores. Chem Rev 123:2737–2831
Marbach S, Bocquet L (2019) Osmosis, from molecular insights to large-scale applications. Chem Soc Rev 48:3102–3144
Behroozi AH, Vatanpour V, Meunier L, Mehrabi M, Koupaie EH (2023) Membrane fabrication and functionalization by atomic layer deposition: processes and applications in water treatment and gas separation. ACS Appl Mater Interfaces 15:13825–13843
Risplendi F, Raffone F, Lin LC, Grossman JC, Cicero G (2020) Fundamental insights on hydration environment of boric acid and its role in separation from saline water. J Phys Chem C 124:1438–1445
Lim YJ, Goh K, Wang R (2022) The coming of age of water channels for separation membranes: from biological to biomimetic to synthetic. Chem Soc Rev 51:4537–4582
Kavokine N, Bocquet ML, Bocquet L (2022) Fluctuation-induced quantum friction in nanoscale water flows. Nature 602:84–90
Lynch CI, Rao SL, Sansom MSP (2020) Water in nanopores and biological channels: a molecular simulation perspective. Chem Rev 120:10298–10335
Radha B, Esfandiar A, Wang FC et al (2016) Molecular transport through capillaries made with atomic-scale precision. Nature 538:222–225
Keerthi A, Geim AK, Janardanan A et al (2018) Ballistic molecular transport through two-dimensional channels. Nature 558:420–424
Dai HW, Xu ZJ, Yang XN (2016) Water permeation and ion rejection in layer−by−layer stacked graphene oxide nanochannels: a molecular dynamics simulation. J Phys Chem C 120:22585–22596
Zhou KG, Vasu KS, Cherian CT et al (2018) Electrically controlled water permeation through graphene oxide membranes. Nature 559:236–240
Yang L, Xiao X, Shen S et al (2022) Recent advances in graphene oxide membranes for nanofiltration. ACS Appl Nano Mater 5:3121–3145
Abraham J, Vasu KS, Williams CD et al (2017) Tunable sieving of ions using graphene oxide membranes. Nat Nanotechnol 12:546–550
Ansari P, Azamat J, Khataee A (2019) Separation of perchlorates from aqueous solution using functionalized graphene oxide nanosheets: a computational study. J Mater Sci 54:2289–2299. https://doi.org/10.1007/s10853-018-3045-2
Qiu RS, Xiao J, Chen XD, Selomulya C, Zhang XW, Woo MW (2020) Relationship between desalination performance of graphene oxide membranes and edge functional groups. ACS Appl Mater Interfaces 12:4769–4776
Shi YX, Li C, He DF, Shen LM, Bao NZ (2017) Preparation of graphene oxide–cellulose acetate nanocomposite membrane for high-flux desalination. J Mater Sci 52:13296–13306. https://doi.org/10.1007/s10853-017-1403-0
Yang JL, Pang YS, Huang WX et al (2017) Functionalized graphene enables highly efficient solar thermal steam generation. ACS Nano 11:5510–5518
Georgakilas V, Tiwari JN, Kemp KC, Perman JA, Bourlinos AB, Kim KS, Zboril R (2016) Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev 116:5464–5519
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Berendsen HJC, Grigera JR, Straatsma TP (1987) The missing term in effective pair potentials. J Phys Chem 91:6269–6271
Duan Y, Wu C, Chowdhury S et al (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem 24:1999–2012
Salman S, Zhao YZ, Zhang XK, Su JY (2020) Effect of temperature on the coupling transport of water and ions through a carbon nanotube in an electric field. J Chem Phys 153:184503
Zhang XK, Li S, Gao SW, Su JY (2023) Bidirectional transport phenomenon of ions in electric fields due to the cluster formation in two-dimensional graphene channels. J Phys Chem C 127:1167–1175
Schmid N, Eichenberger AP, Choutko A, Riniker S, Winger M, Mark AE, van Gunsteren WF (2011) Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur Biophys J 40:843–856
Essmann U, Perera L, Darden T, Berkowitz ML, Lee H, Pedersen LG (1995) A smooth particle mesh ewald method. J Chem Phys 103:8577–8593
Razmkhah M, Ahmadpour A, Mosavian MTH, Moosavi F (2017) What is the effect of carbon nanotube shape on desalination process? A simulation approach. Desalination 407:103–115
Geise GM, Park HB, Sagle AC, Freeman BD, McGrath JE (2011) Water ermeability and water/salt selectivity tradeoff in polymers for desalination. J Membr Sci 369:130–138
Zhao YZ, Huang DC, Su JY, Gao SW (2020) Coupled transport of water and ions through graphene nanochannels. J Phys Chem C 124:17320–17330
Samoylova ON, Calixte EI, Shuford KL (2017) Selective ion transport in functionalized carbon nanotubes. Appl Surf Sci 423:154–159
Ding CX, Zhao YZ, Su JY (2023) Promoting desalination performance of carbon nanotubes through cationic and anionic surface functionalizations. Appl Surf Sci 607:154971
Zhu Y, Wang F, Wu H (2017) Structural and dynamic characteristics in monolayer square ice. J Chem Phys 147:044706
Kaufman IK, Mcclintock PVE (2016) Ionic coulomb blockade. Nature Mater 15:825–826
Ding CX, Zhao YZ, Su JY (2021) Electropumping phenomenon in functionalized carbon nanotubes. Langmuir 37:12318–12326
Zhao YZ, Su JY (2018) Coupling transport of water and ions through a carbon nanotube in a pressure difference: the relation between dynamics and ion structures. J Phys Chem C 122:22178–22187
Sun CZ, Zhou RF, Zhao ZX, Bai BF (2020) Nanoconfined fluids: What can we expect from them? J Phys Chem Lett 11:4678–4692
Secchi E, Marbach S, Nigu`es A, Stein D, Siria A, Bocquet L, (2016) Massive radius-dependent flow slippage in carbon nanotubes. Nature 537:210–213
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) under Grant No. 21873049, 21574066 and the Fundamental Research Funds for Central Universities (FRFCU) under Grant No. 30920021150.
Funding
National Natural Science Foundation of China, 21873049, Jiaye Su, 21574066, Jiaye Su, Fundamental Research Funds for the Central Universities, 30920021150, Jiaye Su
Author information
Authors and Affiliations
Contributions
ZW contributed to the conceptualization, methodology, calculations and writing—original draft. KY contributed to the conceptualization, methodology, calculations and writing—original draft. SL contributed to the conceptualization, methodology, calculations and writing—original draft. XZ contributed to the conceptualization, methodology, calculations and writing—original draft. JS contributed to the conceptualization, methodology, calculations, writing—original draft and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
No experiments involving human participants and/or animals were carried out.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Yang, K., Li, S. et al. Ionic transport through multilayer functionalized graphene channels. J Mater Sci 58, 17303–17312 (2023). https://doi.org/10.1007/s10853-023-09113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-09113-y