Abstract
Today, skin wound healing is still a huge challenge, and how to effectively repair wounds has become a pressing issue. Hydrogel is a very promising treatment. In this study, we prepared a temperature-sensitive hydrogel by loading the active factor taxifolin (TAX) into poloxamer 407, chitosan (CS), and hyaluronic acid (HA). Scanning electron microscopy, X diffraction, and infrared spectroscopy revealed that taxifolin was successfully loaded in the temperature-sensitive hydrogel (TAX-gel). The cross-linked hydrogels exhibited better thermal stability, antioxidant activity, slow release, and safety. In addition, taxifolin composite hydrogel could accelerate the repair of skin damage in mice, and the analysis of the mechanism may be related to the activation of MAPK-mediated autophagy and the increase in VEGF, Pan-Keratin, and CD31 protein expression levels. In this experiment, by constructing a taxifolin temperature-sensitive hydrogel and revealing its mechanism of accelerating skin repair, we fill the gap of taxifolin temperature-sensitive hydrogel and lay the foundation for the development of clinical medical dressing products.
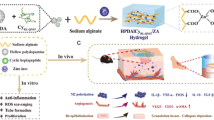
Graphical abstract

TAX-gel accelerates skin wound repair in mice.






Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available and are available from the corresponding author upon reasonable request.
References
Thu HE, Zulfakar MH, Ng SF (2012) Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int J Pharm 434(1–2):375–383
Clark RA, Tonnesen MG, Gailit J, Cheresh DA (1996) Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. Am J Pathol 148(5):1407–1421
Wei Q, Zhang Z, Luo J, Kong J, Ding Y, Chen Y, Wang K (2019) Insulin treatment enhances pseudomonas aeruginosa biofilm formation by increasing intracellular cyclic di-GMP levels, leading to chronic wound infection and delayed wound healing. Am J Transl Res 11(6):3261–3279
Wang F, Zhang C, Dai L, Zhang Y, Wang Y, Hao Y, Ji S, Xu Z, Han N, Chen H, Zhang Q, Nan W (2020) Bafilomycin A1 accelerates chronic refractory wound healing in db/db mice. Biomed Res Int 2020:6265701
Tubbs RS, Shoja MM, Loukas M, Oakes WJ, Ibn Zakaria Razi AM (2007) Rhazes (865-925 AD:) Child's nervous system: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 23(11).
Ullah A, Mamun AA, Zaidi MB, Roome T, Hasan A (2023) A calcium peroxide incorporated oxygen releasing chitosan-PVA patch for Diabetic wound healing. Biomed Pharmacother 165:115156
Li Y, Zhang Y, Wang Y, Yu K, Hu E, Lu F, Shang S, Xie R, Lan G (2022) Regulating wound moisture for accelerated healing: a strategy for the continuous drainage of wound exudates by mimicking plant transpiration. Chem Eng J 429:131964
Wang Y, Liu S, Yu W (2021) Functionalized graphene oxide-reinforced chitosan hydrogel as biomimetic dressing for wound healing. Macromol Biosci 21(4):e2000432
Hui Q, Zhang L, Yang X, Yu B, Huang Z, Pang S, Zhou Q, Yang R, Li W, Hu L, Li X, Cao G, Wang X (2018) Higher biostability of rh-aFGF-Carbomer 940 Hydrogel and its effect on wound healing in a diabetic rat model. Acs Biomater Sci Eng 4(5):1661–1668
Tsai CY, Woung LC, Yen JC, Tseng PC, Chiou SH, Sung YJ, Liu KT, Cheng YH (2016) Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr Polym 135:308–315
Salehi M, Ehterami A, Farzamfar S, Vaez A, Ebrahimi-Barough S (2021) Accelerating healing of excisional wound with alginate hydrogel containing naringenin in rat model. Drug Deliv Transl Res 11(1):142–153
Xu F, Corbett B, Bell S, Zhang C, Budi HM, Farsangi ZJ, Macgregor J, Hoare T (2020) High-throughput synthesis, analysis, and optimization of injectable hydrogels for protein delivery. Biomacromolecules 21(1):214–229
Deng A, Kang X, Zhang J, Yang Y, Yang S (2017) Enhanced gelation of chitosan/beta-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated. Mater Sci Eng C-Mater Biol Appl 78:1147–1154
Wu J, Zhu J, He C, Xiao Z, Ye J, Li Y, Chen A, Zhang H, Li X, Lin L, Zhao Y, Zheng J, Xiao J (2016) Comparative study of heparin-poloxamer hydrogel modified bFGF and aFGF for in vivo wound healing efficiency. Acs Appl Mater Interfaces 8(29):18710–18721
Sultan AA, El-Gizawy SA, Osman MA, El MG (2017) Self dispersing mixed micelles forming systems for enhanced dissolution and intestinal permeability of hydrochlorothiazide. Colloid Sur. B-Biointerfaces 149:206–216
Kim H, Kang H, Kim M, Han S (2018) The effects of barrier agents in postoperative pelvic adhesion formation: a comparative study of a temperature-sensitive poloxamer-based solution/gel and a hyaluronic acid-based solution in a rat uterine horn model. J laparoendosc Adv Surg Tech A 28(2).
Rencber S, Karavana SY, Senyigit ZA, Erac B, Limoncu MH, Baloglu E (2017) Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: formulation, preparation, and in vitro/in vivo evaluation. Pharm Dev Technol 22(4):551–561
Zmejkoski DZ, Markovic ZM, Budimir MD, Zdravkovic NM, Trisic DD, Bugarova N, Danko M, Kozyrovska NO, Spitalsky Z, Kleinova A, Kuzman SB, Pavlovic VB, Todorovic MB (2021) Photoactive and antioxidant nanochitosan dots/biocellulose hydrogels for wound healing treatment. Mater Sci Eng C-Mater Biol Appl 122:111925
Sahu A, Jeon J, Lee MS, Yang HS, Tae G (2021) Antioxidant and anti-inflammatory activities of Prussian blue nanozyme promotes full-thickness skin wound healing. Mater Sci Eng C-Mater Biol Appl 119:111596
Zhou Y, Zhang XL, Lu ST, Zhang NY, Zhang HJ, Zhang J, Zhang J (2022) Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther 13(1):407
Liu H, Zhao Y, Zou Y, Huang W, Zhu L, Liu F, Wang D, Guo K, Hu J, Chen J, Ye L, Li X, Lin L (2019) Heparin-poloxamer hydrogel-encapsulated rhFGF21 enhances wound healing in diabetic mice. Faseb J 33(9):9858–9870
Wallace SN, Carrier DJ, Clausen EC (2005) Batch solvent extraction of flavanolignans from milk thistle (Silybum marianum L. Gaertner). Phytochem Anal 16(1):7–16.
Kiehlmann E, Slade PW (2003) Methylation of dihydroquercetin acetates: synthesis of 5-O-methyldihydroquercetin. J Nat Prod 66(12):1562–1566
Michael N, Robert S (1999) The complete German Commission E monographs: therapeutic guide to herbal medicines. Ann Intern Med 130(5).
Liu X, Zhao Y, Zhu H, Wu M, Zheng Y, Yang M, Cheng Z, Ding C, Liu W (2021) Taxifolin retards the D-galactose-induced aging process through inhibiting Nrf2-mediated oxidative stress and regulating the gut microbiota in mice. Food Funct 12:12142–12158
Zhang J, Chen K, Ding C, Sun S, Zheng Y, Ding Q, Hong B, Liu W (2022) Fabrication of chitosan/PVP/dihydroquercetin nanocomposite film for in vitro and in vivo evaluation of wound healing. Int J Biol Macromol 206:591–604
Wang Y, Ding C, Zhao Y, Zhang J, Ding Q, Zhang S, Wang N, Yang J, Xi S, Zhao T, Zhao C, Liu W (2023) Sodium alginate/poly(vinyl alcohol)/taxifolin nanofiber mat promoting diabetic wound healing by modulating the inflammatory response, angiogenesis, and skin flora. Int J Biol Macromol 252:126530
Liu X, Zhao Y, Chen X, Dong L, Zheng Y, Wu M, Ding Q, Xu S, Ding C, Liu W (2021) Establishment of callus induction system, histological evaluation and taxifolin production of Larch. Plant Cell Tissue Organ Culture (PCTOC) 147(3).
Liang J, Zhang K, Li J, Su J, Guan F, Li J (2022) Injectable protocatechuic acid based composite hydrogel with hemostatic and antioxidant properties for skin regeneration. Mater Des 222:111109
Coppari S, Colomba M, Fraternale D, Brinkmann V, Romeo M, Rocchi M, Di Giacomo B, Mari M, Guidi L, Ramakrishna S, Ventura N, Albertini MC (2021) Antioxidant and anti-inflammaging ability of prune (Prunus spinosa L.) extract result in improved wound healing efficacy. Antioxidants 10(3).
Numata Y, Terui T, Okuyama R, Hirasawa N, Sugiura Y, Miyoshi I, Watanabe T, Kuramasu A, Tagami H, Ohtsu H (2006) The accelerating effect of histamine on the cutaneous wound-healing process through the action of basic fibroblast growth factor. J Invest Dermatol 126(6):1403–1409
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6(4):389–395
Gonzalez ACDO, Costa TF, Andrade ZDA, Medrado ARAP (2016) Wound healing—a literature review. An Brasil Dermatol 91(5).
Choi MS, Chae YJ, Choi JW, Chang JE (2021) Potential therapeutic approaches through modulating the autophagy process for skin barrier dysfunction. Int J Mol Sci 22(15).
Wang M, Lei M, Chang L, Xing Y, Guo Y, Pourzand C, Bartsch JW, Chen J, Luo J, Widyakarisma V, Nisar MF, Lei X, Zhong JL (2021) Bach2 regulates autophagy to modulate UVA-induced photoaging in skin fibroblasts. Free Radic Biol Med 169:304–316
Riffelmacher T, Richter FC, Simon AK (2018) Autophagy dictates metabolism and differentiation of inflammatory immune cells. Autophagy 14(2):199–206
Chen JC, Lin BB, Hu HW, Lin C, Jin WY, Zhang FB, Zhu YA, Lu CJ, Wei XJ, Chen RJ (2014) NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways. Biomed Res Int 2014:547187
Sil P, Muse G, Martinez J (2018) A ravenous defense: canonical and non-canonical autophagy in immunity. Curr Opin Immunol 50:21–31
Fang D, Xie H, Hu T, Shan H, Li M (2021) Binding features and functions of ATG3. Front Cell Dev Biol 9:685625
Acknowledgements
This study was supported by grants from the Jilin Province Science and Technology Development Program (Grant No. 20220508062RC) and the Jilin Agricultural Science and Technology College Doctoral starting fund [Grant No. (2022) 738].
Author information
Authors and Affiliations
Contributions
All authors made equal contributions to researching data, discussion, and analysis of the content of the manuscript. CD contributed to writing—original draft, visualization, data curation, investigation, funding acquisition. ZL contributed to visualization. TZ contributed to visualization, methodology, investigation, data curation, formal analysis. XL contributed to writing—review & editing. LM contributed to supervision. JZ contributed to supervision. MY contributed to data curation, investigation. SS contributed to conceptualization, funding acquisition, supervision, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This study was approved by the animal research ethics committee of Jilin Agricultural University, ethics approval No.: 2022-08-28-002.
Additional information
Handling Editor: Steven Naleway.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, C., Liu, Z., Zhao, T. et al. A temperature-sensitive hydrogel loaded with taxifolin promotes skin repair by modulating MAPK-mediated autophagic pathway. J Mater Sci 58, 14831–14845 (2023). https://doi.org/10.1007/s10853-023-08951-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08951-0