Abstract
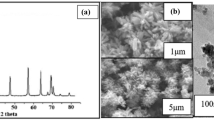
Natural bioactive compounds exhibit potent and promising antibacterial activity against Multidrug resistant (MDR) bacteria by cell wall permeation and inhibiting the efflux pump system. This research work deals with the fabrication of natural bioactive compound and commercially available antibiotic drug-loaded metal nanoparticle-based polymeric nanocarrier for the treatment of MDR bacterial infections. Initially, the vancomycin-loaded citrate-ZnO was prepared, followed by the chitosan-coated vancomycin-citrate-ZnO carrier. Further, curcumin was loaded in the chitosan-vancomycin-citrate-ZnO carrier (Cur-Chit-Van-Cit-ZnO). The synthesized carriers were characterized using UV-visible spectroscopy, FTIR, XRD, DLS, SEM, and HR-TEM analysis. The elements present in the carriers were evaluated using EDX analysis. Biological studies were carried out to examine the antibacterial activity of ZnO, carrier, and drug-loaded carriers using disk diffusion and MIC analysis. The XRD results indicate the amorphous nature of Cur-Chit-Van-Cit-ZnO, and the surface morphological studies exhibit a spherical-like structure. The presence of Zn was evaluated from EDX analysis as 28% in Cur-Chit-Van-Cit-ZnO. Disk diffusion analysis exhibits potential antibacterial activity for Cur-Chit-Van-Cit-ZnO against Methicillin-resistant Staphylococcus aureus (MRSA) and K. pneumonia with 22 mm and 14 mm of zone of inhibition measurements. The drug-loaded carrier showed 112 μg/mL and 150 μg/mL concentrations as MIC values against MRSA and K. pneumonia. The physicochemical characterizations and biological analyses were confirmed the synthesized drug-loaded carrier’s formation and inhibition efficiency against MRSA and K. pneumonia. It will provide a potential formulation against MDR bacterial infections.






Similar content being viewed by others
References
Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S (2015) A new antibiotic kills pathogens without detectable resistance. Nature 517:455–459
Khameneh B, Diab R, Ghazvini K, Bazzaz BSF (2016) Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog 95:32–42
Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ (2015) Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51
Gordon RJ, Lowy FD (2008) Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:S350–S359
Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S (2013) National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 173:1970–1978
Mukheem A, Muthoosamy K, Manickam S, Sudesh K, Shahabuddin S, Saidur R, Akbar N, Sridewi N (2018) Fabrication and characterization of an electrospun PHA/graphene silver nanocomposite scaffold for antibacterial applications. Materials 11:1673
Siddiqi KS, Ur Rahman A, Tajuddin HA (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett 13:141
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227–1249
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett 7:219–242
Souza VGL, Rodrigues C, Valente S, Pimenta C, Pires JRA, Alves MM, Santos CF, Coelhoso IM, Fernando AL (2020) Eco-friendly ZnO/chitosan bionanocomposites films for packaging of fresh poultry meat. Coatings 10:110
Liu Y, Shen L, Lin H, Yu W, Xu Y, Li R, Sun T, He Y (2020) A novel strategy based on magnetic field assisted preparation of magnetic and photocatalytic membranes with improved performance. J Membr Sci 612:118378
Shen L, Huang Z, Liu Y, Li R, Xu Y, Jakaj G, Lin H (2020) Polymeric Membranes incorporated with ZnO nanoparticles for membrane fouling mitigation: a brief review. Front Chem 8:224
Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R, Cano A, Espina M, Ettcheto M, Camins A et al (2020) Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10:292
Giau VV, An SSA, Hulme J (2019) Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des Dev Ther 13:327–343
Zhao D, Yu S, Sun B, Gao S, Guo S, Zhao K (2018) Biomedical applications of chitosan and its derivative nanoparticles. Polymers (Basel) 10(4):462
Mohebbi S, Nezhad MN, Zarrintaj P, Jafari SH, Gholizadeh SS, Saeb MR, Mozafari M (2019) Chitosan in biomedical engineering: a critical review. Curr Stem Cell Res Ther 14:93–116
Bagheri B, Zarrintaj P, Surwase SS, Saeb MR, Mozafari M, Park OO, Kim YC (2019) Self-gelling electroactive hydrogels based on chitosan-aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Coll Surf B: Biointerfaces 184:110549
Shalumon KT, Binulal NS, Selvamurugan N, Nair SV, Menon D, Furuike T, Tamura H, Jayakumar R (2009) Electrospinning of carboxymethyl chitin/poly(vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. Carbohydr Polym 77:863–869
Neu HC (1992) The crisis in antibiotic resistance. Science 257:1064–1073
Portes E, Gardrat C, Castellan A, Coma V (2009) Environmentally friendly films based on chitosan and tetrahydrocurcuminoid derivatives exhibiting antibacterial and antioxidative properties. Carbohydr Polym 76:578–584
Sonkaew P, Sane A, Suppakul P (2012) Antioxidant activities of curcumin and ascorbyldipalmitate nanoparticles and their activities after incorporation into cellulose-based packaging films. J Agric Food Chem 60:5388–5399
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23:363–398
Kant V, Gopal A, Pathak NN, Kumar P, Tandan SK, Kumar D (2014) Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int Immunopharmacol 20:322–330
Ruby AJ, Kuttan G, Dinesh BK, Rajasekharan KN, Kuttan R (1995) Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 94:79–83
Tängdén T (2014) Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Upsala J Med Sci 119:149–153
Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M (2012) Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950
Cong Y, Yang S, Rao X (2019) Vancomycin resistant Staphylococcus aureus infections: a review of case updating and clinical features. J Adv Res 21:169–176
Mun SH, Kim SB, Kong R, Choi JG, Kim YC, Shin DW, Kang OH, Kwon DY (2014) Curcumin reverse methicillin resistance in Staphylococcus aureus. Molecules 19:18283–18295
Akbar N, Aslam Z, Siddiqui R, Shah MR, Khan NA (2021) Zinc oxide nanoparticles conjugated with clinically approved medicines as potential antibacterial molecules. AMB Expr 11:104
Thottoli AK, Unni AKA (2013) Effect of trisodium citrate concentration on the particle growth of ZnS nanoparticles. J Nanostr Chem 3:56
Bao Y, Wang C, Ma JZ (2016) Morphology control of ZnO microstructures by varying hexamethylenetetramine and trisodium citrate concentration and their photocatalytic activity. Mater Des 101:7–15
Bao Y, Wang C, Ma J (2016) Trisodium citrate as bridging and suppressing agent to control synthesis of ZnO hollow hierarchical microspheres and their photocatalytic properties. Ceram Int 42:1746–1755
Kaur A, Preet S, Kumar V, Kumar R, Kumar R (2019) Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Coll Surf B: Biointerfaces 176:62–69
Mohapatra A, Harris MA, Le Vine D, Ghimire M, Jennings JA, Morshed BI, Haggard WO, Bumgardner JD, Mishra SR, Fujiwara T (2017) Magnetic stimulus responsive vancomycin drug delivery system based on chitosan microbeads embedded with magnetic nanoparticles. J Biomed Mater Res Part B 106(6):2169–2176
Xu J, Xu B, Shou D, Xia X, Hu Y (2015) Preparation and evaluation of vancomycin-loaded n-trimethyl chitosan nanoparticles. Polymers 7:1850–1870
Finotelli PV, Silva DD, Sola-Penna M, Rossi AM, Farina M, Andrade LR, Takeuchi AY, Rocha-Leǎo MH (2010) Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin. Coll Surf B: Biointerfaces 81:206–211
Liu Y, Cai Y, Jiang X, Wu J, Le X (2016) Molecular interactions, characterization and antimicrobial activity of curcumin-chitosan blend films. Food Hydrocoll 52:564–572
Sonkaew P, Sane A, Suppakul P (2012) Antioxidant activities of curcumin and ascorbyl dipalmitate nanoparticles and their activities after incorporation into cellulose-based packaging films. J Agric Food Chem 60:5388–5399
Meva FE, Segnou ML, Ebongue CO, Ntoumba AA, Kedi PBE, Deli V, Etoh MA, Mpondo EM (2016) Spectroscopic synthetic optimizations monitoring of silver nanoparticles formation from Megaphrynium macrostachyum leaf extract. Rev Bras Farmacogn 26:640–646
Pan Z, Zeng B, Yu G, Teng J, Zhang H, Shen L, Yang L, Lin H (2022) Mechanistic insights into Ca-alginate gel-associated membrane fouling affected by ethylene diamine tetraacetic acid (EDTA). Sci Total Environ 842:156912
Long Y, Yu G, Dong L, Xu Y, Lin H, Deng Y, You X, Yang L, Liao BQ (2021) Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res 189:116665
Chandran S, Ravichandran V, Chandran S, Chemmanda J, Chandrasekar B (2016) Biosynthesis of PVA encapsulated silver nanoparticles. JART 14:319–324
Xu S, Han Y, Zhou C, Li J, Shen L, Lin H (2023) A biobased flame retardant towards improvement of flame retardancy and mechanical property of ethylene vinyl acetate. Chin Chem Lett 34:107202
Han L, Chen C, Shen L, Lin H, Li B, Huang Z, Xu Y, Li R, Hong H (2022) Novel membranes with extremely high permeability fabricated by 3D printing and nickel coating for oil/water separation. J Mater Chem A 10:12055–12061
Ahmed S, Saifullah AM, Swami BL, Ikram S (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. JRRAS 9:1–7
Gan Y, Gu F, Han D, Wang Z, Guo G (2010) Biomimetic synthesis of zinc oxide 3D architectures with gelatin as matrix. J Nanomater 2010:289173
Rauf MA, Owais M, Rajpoot R, Ahmad F, Khan N, Zubair S (2017) Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible S. aureus skin infection in experimental animals. RSC Adv 7:36361–36373
Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH (2008) Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Environ Microbiol 74:2171–2178
Shahbazi N, Zare-Dorabei R (2019) A facile colorimetric and spectrophotometric method for sensitive determination of metformin in human serum based on citrate-capped gold nanoparticles: central composite design optimization. ACS Omega 4:17519–17526
Jin C, Liu X, Tan L, Cui Z, Yang X, Zheng Y, Yeung KWK, Chu PK, Wu S (2018) Ag/AgBr-loaded mesoporous silica for rapid sterilization and promotion of wound healing. Biomater Sci 6:1735–1744
Xie X, Mao C, Liu X, Tan L, Cui Z, Yang X, Zhu S, Li Z, Yuan X, Zheng Y, Yeung KWK, Chu PK, Wu S (2018) Tuning the band-gap of photo-sensitive polydopamine/Ag3PO4/graphene oxide coating for rapid, noninvasive disinfection of implants. ACS Cent Sci 4:724–738
Costa LMD, Reynolds PE, Souza HA, Souza DC, Palepou MF, Woodford N (2000) Characterization of a divergent vanD-type resistance element from the first glycopeptide-resistant strain of Enterococcus faecium isolated in Brazil. Antimicrob Agents Chemother 44:3444–3446
Joseph WN, Moyu W, Alejandra DB, Dejian Z, Manuel V, Matthew B (2008) Nanomechanical detection of antibiotic-mucopeptide binding in a model for superbug drug resistance. Nat Nanotechnol 3:691–696
Preet S, Verma I, Rishi P (2010) Cryptdin-2: a novel therapeutic agent for experimental Salmonella typhimurium infection. J Antimicrob Chemother 65:991–994
Kabiriyel J, Jeyanthi R, Jayakumar K, Amalraj A, Arjun P, Shanmugarathinam A, Vignesh G, Mohan CR (2023) Green synthesis of carboxy methyl chitosan based curcumin nanoparticles and its biological activity: Influence of size and conductivity. Carbohydr Polym Technol Appl 5:100260
Rishi P, Preet S, Bharrhan S, Verma I (2011) In vitro and in vivo synergistic effects of cryptdin 2 and ampicillin against salmonella. Antimicrob Agents Chemother 55:4176–4182
Brause R, Moltgen H, Kleinermanns K (2011) Characterization of laser ablated and chemically reduced silver colloids in aqueous solution by UV/VIS spectroscopy and STM/SEM microscopy. Appl Phys B 75:711–716
Kaur A, Goyal D, Kumar R (2018) Surfactant mediated interaction of vancomycin with silver nanoparticles. Appl Surf Sci 449:23–30
Wang J, Zhu R, Sun X, Zhu Y, Liu H, Wang SL (2014) Intracellular uptake of etoposide loaded solid lipid nanoparticles induces an enhancing inhibitory effect on gastric cancer through mitochondria-mediated apoptosis pathway. Int J Nanomed 9:3987–3998
Lundstrom TS, Sobel JD (2000) Antibiotics for gram-positive bacterial infections: vancomycin, teicoplanin, quinupristin/dalfopristin, and linezolid. Infect Dis Clin 14:463–474
Vimala K, Mohan YM, Varaprasad K, Redd NN, Ravindra S, Naidu NS, Raju KM (2011) Fabrication of curcumin encapsulated chitosan-PVA silver nanocomposite films for improved antimicrobial activity. J Biomater Nanobiotechnol 2:55–64
Purwaningsih SY, Pratapa S, Triwikantoro D (2016) Nano-sized ZnO powders prepared by co-precipitation method with various pH. AIP Conf Proc 1725:020063
Kamaruzaman A, Lah NAC (2021) Morphological changes of ZnO nanostructures upon addition of trisodium citrate (Na3C6H5O7) at different reaction temperatures. JMMST 5:18–22
Gopinath V, Priyadarshini S, Loke MF, Arunkumar J, Marsili E, Mubarak Ali D, Velusamy P, Vadivelu J (2015) Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab J Chem 11:1878–5352
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275
Mfouo-Tynga I, El-Hussein A, Abdel-Harith M, Abrahamse H (2014) Photodynamic ability of silver nanoparticles in inducing cytotoxic effects in breast and lung cancer cell lines. Int J Nanomed Nanosurg 9:3771–3780
Dananjaya SHS, Kumar RS, Yang M, Nikapitiya C, Lee J, De Zoysa M (2018) Synthesis, characterization of ZnO-chitosan nanocomposites and evaluation of its antifungal activity against pathogenic Candida albicans. Int J Biol Macromol 108:1281–1288
Mubeen B, Ansar AN, Rasool R, Ullah I, Imam SS, Alshehri S, Ghoneim MM, Alzarea SI, Nadeem MS, Kazmi I (2021) Nanotechnology as a novel approach in combating microbes providing an alternative to antibiotics. Antibiotics 10:1473
Sarkar P, Yarlagadda V, Ghosh C, Haldar J (2017) A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Medchemcomm 8:516–533
Teow SY, Liew K, Ali SA, Khoo ASB, Peh SC (2016) Antibacterial action of curcumin against Staphylococcus aureus: a brief review. J Trop Med 2016:1–10
Praphakar RA, Ebenezer RS, Vignesh S, Shakila H, Rajan M (2019) Versatile pH-responsive chitosan-g polycaprolactone/maleic anhydride-isoniazid polymeric micelle to improve the bioavailability of tuberculosis multidrugs. ACS Appl Bio Mater 2:1931–1943
Acknowledgements
M. Rajan acknowledges significant financial support from the plan of the Science and Engineering Research Board (Ref: EEQ/2020/000201) New Delhi, India and Rashtriya Uchchatar Shiksha Abhiyan (RUSA), Madurai Kamaraj University, (File No. 007-R2/RUSA/MKU/2020-2021) and also acknowledges the PURSE program for the purchase of SEM and FTIR, and UPE programs for the purchase of TEM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nandhini, P., Ramya, R.D., Murugan, M. et al. Vancomycin and curcumin-loaded zinc oxide functionalized chitosan carrier for the treatment of multi-drug resistant bacterial infection. J Mater Sci 58, 4922–4936 (2023). https://doi.org/10.1007/s10853-023-08290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08290-0