Abstract
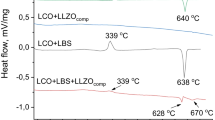
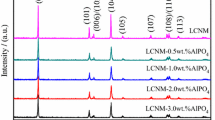
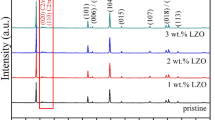
The critical issue of high resistance at the interface between cathode and solid electrolyte for creating all-solid-state power sources can be addressed by introducing a low-melting additive (Li3BO3) and lithium-conducting solid electrolyte (Li7La3Zr2O12) in the LiNi1/3Co1/3Mn1/3O2 cathode mass. The chemical and thermal stability of the solid electrolyte in contact with LiNi1/3Co1/3Mn1/3O2 and Li3BO3 was studied using XRD and DSC analysis. It was found that the introduction of 5 wt% Li3BO3 in LiNi1/3Co1/3Mn1/3O2 leads to a close contact between the solid electrolyte and cathode and a decrease in the interfacial resistance from 45000 to 85 Ω cm2 at 300 °C compared to pure LiNi1/3Co1/3Mn1/3O2. The addition of 5 wt% lithium-conductive electrolyte to the cathode mass does not lead to significant changes in interface resistance. No degradation processes in the components of the experimental cell with composite cathode and Li anode were found during electrochemical experiments.
Graphical abstract














Similar content being viewed by others
References
Bates AM, Preger Y, Torres-Castro L, Harrison KL, Harris SJ, Hewson J (2022) Are solid-state batteries safer than lithium-ion batteries? Joule 6(4):742–755. https://doi.org/10.1016/j.joule.2022.02.007
Xu L, Lu Y, Zhao C-Z, Yuan H, Zhu G-L, Hou L-P, Zhang Q, Huang J-Q (2021) Toward the scale-up of solid-state lithium metal batteries: the gaps between lab-level cells and practical large-format batteries. Adv Energy Mater 11:2002360. https://doi.org/10.1002/aenm.202002360
Kong L, Wang L, Zhu J, Bian J, Xia W, Zhao R, Lin H, Zhao Y (2021) Configuring solid-state batteries to power electric vehicles: a deliberation on technology, chemistry and energy. ChemComm 57(94):12587–12594. https://doi.org/10.1039/D1CC04368D
Chen L, Huang YF, Ma J, Ling H, Kang F, He YB (2020) Progress and perspective of all-solid-state lithium batteries with high performance at room temperature. Energy Fuels 34:13456–13472. https://doi.org/10.1021/acs.energyfuels.0c02915
Kim JG, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi MJ, Chung HY, Park S (2015) A review of lithium and non-lithium based solid-state batteries. J Power Sources 282:299–322. https://doi.org/10.1016/j.jpowsour.2015.02.054
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386. https://doi.org/10.1016/j.nanoen.2017.01.028
Kammampata SP, Thangadurai V (2018) Cruising in ceramics—discovering new structures for all-solid-state batteries—fundamentals, materials, and performances. Ionics 24:639–660. https://doi.org/10.1007/s11581-017-2372-7
Zheng F, Kotobuki M, Song S, Lai MO, Lu L (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 389:198–213. https://doi.org/10.1016/j.jpowsour.2018.04.022
Takada K (2018) Progress in solid electrolytes toward realizing solid-state lithium batteries. J Power Sources 394:74–85. https://doi.org/10.1016/j.jpowsour.2018.05.003
Yaroslavtsev AB (2016) Solid electrolytes: main prospects of research and development. Rus Chem Rev 85:1255–1276. https://doi.org/10.1070/RCR4634/pdf
Murugan R, Thangadurai V, Weppner W (2007) Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed 46:7778–7781. https://doi.org/10.1002/anie.200701144
Ramakumar S, Deviannapoorani C, Dhivya L, Shankar LS, Murugan R (2017) Lithium garnets: synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog Mater Sci 88:325–411. https://doi.org/10.1016/j.pmatsci.2017.04.007
Il’ina E, Lylin E, Vlasov M, Kabanov A, Okhotnikov K, Sherstobitova E, Zobel M (2022) Structural features and Li-ion diffusion mechanism in tantalum-doped Li7La3Zr2O12 solid electrolytes. ACS Appl Energy Mater 5(3):2959–2967. https://doi.org/10.1021/acsaem.1c03632
Li Y, Wang CA, Xie H, Cheng J, Goodenough JB (2011) High lithium ion conduction in garnet-type Li6La3ZrTaO12. Electrochem Comm 13:1289–1292. https://doi.org/10.1016/j.elecom.2011.07.008
Li Y, Han JT, Wang CA, Xie H, Goodenough JB (2012) Optimizing Li+ conductivity in a garnet framework. J Mater Chem 22:15357–15361. https://doi.org/10.1039/c2jm31413d
Antipov EV, Abakumov AM, Drozhzhin OA, Pogozhev DV (2019) Lithium-ion electrochemical energy storage: the current state, problems, and development trends in russia. Therm Eng 66:219–224. https://doi.org/10.1134/S0040601519040013
Zhu L, Bao C, Xie L, Yang X, Cao X (2020) Review of synthesis and structural optimization of LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium-ion batteries applications. J Alloys Compd 831:154864. https://doi.org/10.1016/j.jallcom.2020.154864
Sun H, Zhao K (2017) Electronic structure and comparative properties of LiNixMnyCozO2 cathode materials. J Phys Chem C 121:6002–6010. https://doi.org/10.1021/acs.jpcc.7b00810
Ivanishchev AV, Bobrikov IA, Ivanishcheva IA, Ivanshina OY (2018) Study of structural and electrochemical characteristics of LiNi0.33Mn0.33Co0.33O2 electrode at lithium content variation. J Electroanal Chem 821:140–151. https://doi.org/10.1016/j.jelechem.2018.01.020
Chen R, Li Q, Yu X, Chen L, Li H (2020) Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem Rev 120:6820–6877. https://doi.org/10.1021/acs.chemrev.9b00268
Alexander GV, Indu MS, Murugan R (2021) Review on the critical issues for the realization of all solid state lithium metal batteries with garnet electrolyte: interfacial chemistry, dendrite growth, and critical current densities. Ionics 27:4105–4126. https://doi.org/10.1007/s11581-021-04190-y
Indu MS, Alexander GV, Sreejith OV, Abraham SE, Murugan R (2021) Lithium garnet-cathode interfacial chemistry: inclusive insights and outlook toward practical solid-state lithium metal batteries. Mater Today Energy 21:100804. https://doi.org/10.1016/j.mtener.2021.100804
Il’ina EA, Raskovalov AA (2020) Studying of superionic solid electrolyte Li7La3Zr2O12 stability by means of chemical thermodynamics for application in all-solid-state batteries. Electrochim Acta 330:135220. https://doi.org/10.1016/j.electacta.2019.135220
Alexander GV, Rosero-Navarro NC, Miura A, Tadanaga K, Murugan R (2018) Electrochemical performance of a garnet solid electrolyte based lithium metal battery with interface modification. J Mater Chem A 6(42):21018–21028. https://doi.org/10.1039/C8TA07652A
Li Y, Wang Z, Cao Y, Du F, Cui Z, Guo X (2015) W-doped Li7La3Zr2O12 Ceramic electrolytes for solid State Li-ion batteries. Electrochim Acta 180:37–42. https://doi.org/10.1016/j.electacta.2015.08.046
Ferreira EB, Lima ML, Zanotto ED (2010) DSC method for determining the liquidus temperature of glass-forming systems. J Am Ceram Soc 93:3757–3763. https://doi.org/10.1111/j.1551-2916.2010.03976.x
Il’ina EA, Pershina SV, Antonov BD, Pankratov AA (2021) Impact of Li3BO3 addition on solid electrode - solid electrolyte interface in all-solid-state batteries. Materials 14:7099. https://doi.org/10.3390/ma14227099
Kwatek K, Slubowska W, Ruiz C, Sobrados I, Sanz J, Garbarczyk JE, Nowinski JL (2020) The mechanism of enhanced ionic conductivity in Li1.3Al0.3Ti1.7(PO4)3–(0.75Li2O·0.25B2O3) composites. J Alloys Compd 838:155623. https://doi.org/10.1016/j.jallcom.2020.155623
Acknowledgements
The research has been carried out with the equipment of the Shared Access Center “Composition of Compounds” of the Institute of High Temperature Electrochemistry. This work was supported by RFBR and Sverdlovsk region, project number 20-43-660015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Ethical approval
Not applicable.
Additional information
Handling Editor: Mark Bissett.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Il’ina, E.A., Druzhinin, K.V., Kuznetsova, T.A. et al. Interface modification between Ta, Al-doped Li7La3Zr2O12 solid electrolyte and LiNi1/3Co1/3Mn1/3O2 cathode in all-solid-state batteries. J Mater Sci 58, 4070–4081 (2023). https://doi.org/10.1007/s10853-023-08268-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08268-y