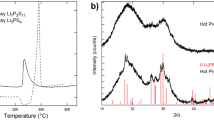
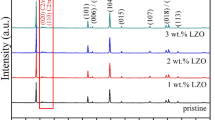
Abstract
Cathode modification by Li2O–B2O3–SiO2 glass addition can be considered one of the ways to solve the problem of the increased interfacial resistance between solid electrolyte and solid cathode. The chemical and thermal stability of composite solid electrolyte based on Li7La3Zr2O12 in contact with LiCoO2 and glass additive was studied using XRD and DSC analysis. It was established that the interaction in the mixture studied begins at 670 °C. Therefore, 650 °C at 1 h was chosen as heat treatment conditions for (100-x)LiCoO2 + x 65Li2O·27B2O3·8SiO2 | solid electrolyte half-cells (x = 0–20 wt%). It was established that 5 wt% of glass addition is optimal to reduce the interfacial resistance. It can be concluded that Li2O–B2O3–SiO2 glass addition in LiCoO2 leads to the tight contact with composite electrolyte based on Li7La3Zr2O12 and the decrease in the interface resistance between the two solids.







Similar content being viewed by others
References
Kim JG, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi MJ, Chung HY, Park S (2015) A review of lithium and non-lithium based solid-state batteries. J Power Sources 282:299–322. https://doi.org/10.1016/j.jpowsour.2015.02.054
Chen L, Huang YF, Ma J, Ling H, Kang F, He YB (2020) Progress and perspective of all-solid-state lithium batteries with high performance at room temperature. Energy Fuel 34:13456–13472. https://doi.org/10.1021/acs.energyfuels.0c02915
Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, Yonemura M, Iba H, Kanno R (2016) High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy 1:16030. https://doi.org/10.1038/NENERGY.2016.30
Chen R, Li Q, Yu X, Chen L, Li H (2020) Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem Rev 120:6820–6877. https://doi.org/10.1021/acs.chemrev.9b00268
Alexander GV, Indu MS, Murugan R (2021) Review on the critical issues for the realization of all-solid-state lithium metal batteries with garnet electrolyte: interfacial chemistry, dendrite growth, and critical current densities. Ionics 27:4105–4126. https://doi.org/10.1007/s11581-021-04190-y
Xu L, Lu Y, Zhao C-Z, Yuan H, Zhu G-L, Hou L-P, Zhang Q, Huang J-Q (2021) Toward the scale-up of solid-state lithium metal batteries: the gaps between lab-level cells and practical large-format batteries. Adv Energy Mater 11:2002360. https://doi.org/10.1002/aenm.202002360
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386. https://doi.org/10.1016/j.nanoen.2017.01.028
Zheng F, Kotobuki M, Song S, Lai MO, Lu L (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 389:198–213. https://doi.org/10.1016/j.jpowsour.2018.04.022
Campanella D, Belanger D, Paolella A (2021) Beyond garnets, phosphates and phosphosulfides solid electrolytes: new ceramic perspectives for all solid lithium metal batteries. J Power Sources 482:228949. https://doi.org/10.1016/j.jpowsour.2020.228949
Xu L, Li J, Deng W, Shuai H, Li S, Xu Z, Li J, Hou H, Peng H, Zou G, Ji X (2021) Garnet solid electrolyte for advanced all-solid-state Li batteries. Adv Energy Mater 11:2000648. https://doi.org/10.1002/aenm.202000648
Samson AJ, Hofstetter K, Baga S, Thangadurai V (2019) A bird’s-eye view of Li-stuffed garnet-type Li7La3Zr2O12 ceramic electrolytes for advanced all-solid-state Li batteries. Energy Environ Sci 12:2957–2975. https://doi.org/10.1039/C9EE01548E
Ramakumar S, Deviannapoorani C, Dhivya L, Shankar LS, Murugan R (2017) Lithium garnets: synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog Mater Sci 88:325–411. https://doi.org/10.1016/j.pmatsci.2017.04.007
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367. https://doi.org/10.1038/35104644
Murugan R, Thangadurai V, Weppner W (2007) Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed 46:7778–7781. https://doi.org/10.1002/anie.200701144
Rosero-Navarro NC, Yamashita T, Miura A, Higuchi M, Tadanaga K, Stevenson JW (2017) Effect of sintering additives on relative density and Li-ion conductivity of Nb-doped Li7La3ZrO12 solid electrolyte. J Am Ceram Soc 100:276–285. https://doi.org/10.1111/jace.14572
Tang Y, Zhang Q, Luo Z, Liu P, Lu A (2017) Effects of Li2O-Al2O3-SiO2 system glass on the microstructure and ionic conductivity of Li7La3Zr2O12 solid electrolyte. Mater Lett 193:251–254. https://doi.org/10.1016/j.matlet.2017.01.134
Il’ina EA, Pershina SV, Antonov BD, Pankratov AA, Vovkotrub EG (2018) The influence of the glass additive Li2O-B2O3-SiO2 on the phase composition, conductivity, and microstructure of the Li7La3Zr2O12. J Alloys Compd 765:841–847. https://doi.org/10.1016/j.jallcom.2018.06.154
Duan H, Zheng H, Zhou Y, Xu B, Liu H (2018) Stability of garnet-type Li ion conductors: an overview. Solid State Ionics 318:45–53. https://doi.org/10.1016/j.ssi.2017.09.018
Xu B, Li W, Duan H, Wang H, Guo Y (2017) Li3PO4-added garnet-type Li6.5La3Zr1.5Ta0.5O12 for Li-dendrite suppression. J Power Sources 354:68–73. https://doi.org/10.1016/j.jpowsour.2017.04.026
Il’ina EA, Druzhinin KV, Antonov BD (2020) Stability of composite electrolytes based on Li7La3Zr2O12 to metallic lithium. Ionics 26:163–172. https://doi.org/10.1007/s11581-019-03177-0
Ding Z, Li J, Li J, An C (2020) Interfaces: key issue to be solved for all solid-state lithium battery technologies. J Electrochem Soc 167:070541. https://doi.org/10.1149/1945-7111/ab7f84
Xiao Y, Wang Y, Bo SH, Kim JC, Miara LJ, Ceder G (2020) Understanding interface stability in solid-state batteries. Nat Rev Mater 5:105–126. https://doi.org/10.1038/s41578-019-0157-5
Indu MS, Alexander GV, Sreejith OV, Abraham SE, Murugan R (2021) Lithium garnet-cathode interfacial chemistry: inclusive insights and outlook toward practical solid-state lithium metal batteries. Mater Today Energy 21:100804. https://doi.org/10.1016/j.mtener.2021.100804
Il’ina EA, Druzhinin KV, Lyalin ED, Antonov BD, Pankratov AA, Pryakhina VI (2021) Influence of Al layer thickness on Li6.6Al0.05La3Zr1.75Nb0.25O12 solid electrolyte | Li anode interface in all-solid-state batteries. Solid State Ionics 370:115736. https://doi.org/10.1016/j.ssi.2021.115736
Alexander GV, Indu MS, Kamakshy S, Murugan R (2020) Development of stable and conductive interface between garnet structured solid electrolyte and lithium metal anode for high performance solid-state battery. Electrochim Acta 332:135511. https://doi.org/10.1016/j.electacta.2019.135511
Alexander GV, Patra S, Raj SVS, Sugumar MK, Ud Din MM, Murugan R (2018) Electrodes-electrolyte interfacial engineering for realizing room temperature lithium metal battery based on garnet structured solid fast Li+ conductors. J Power Sources 396:764–773. https://doi.org/10.1016/j.jpowsour.2018.06.096
Afyon S, Krumeich F, Mensing C, Borgschulte A, Nesper R (2014) New high capacity cathode materials for rechargeable Li-ion batteries: vanadate-borate glasses. Sci Rep 4:1–7. https://doi.org/10.1038/srep07113
Il’ina EA, Druzhinin KV, Saetova NS, Antonov BD, Pryakhina VI (2018) Interface features between 30Li2O·47.5V2O5·22.5B2O3 glassy cathode and Li7La3Zr2O12 solid electrolyte. Electrochim Acta 285:326–335. https://doi.org/10.1016/j.electacta.2018.08.008
Ohta S, Komagata S, Seki J, Saeki T, Morishita S, Asaoka T (2013) All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J Power Sources 238:53–56. https://doi.org/10.1016/j.jpowsour.2013.02.073
Liu T, Ren Y, Shen Y, Zhao S-X, Lin Y, Nan C-W (2016) Achieving high capacity in bulk-type solid-state lithium ion battery based on Li6.75La3Zr1.75Ta0.25O12 electrolyte: Interfacial resistance. J Power Sources 324:349–357. https://doi.org/10.1016/j.jpowsour.2016.05.111
Park K, Yu B-C, Jung J-W, Li Y, Zhou W, Gao H, Son S, Goodenough JB (2016) Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: Interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem Mater 28:8051–8059. https://doi.org/10.1021/acs.chemmater.6b03870
Il’ina EA, Pershina SV, Antonov BD, Pankratov AA (2021) Impact of Li3BO3 addition on solid electrode - solid electrolyte interface in all-solid-state batteries. Materials 14:7099. https://doi.org/10.3390/ma14227099
Saetova NS, Raskovalov AA, Antonov BD, Yaroslavtseva TV, Reznitskikh OG, Kadyrova NI (2016) The influence of lithium oxide concentration on the transport properties of glasses in the Li2O–B2O3–SiO2 system. J Non-Cryst Solids 443:75–81. https://doi.org/10.1016/j.jnoncrysol.2016.04.025
Bensalah N, Dawood H (2016) Review on synthesis, characterizations, and electrochemical properties of cathode materials for lithium ion batteries. J Mater Sci Eng 5:1000258. https://doi.org/10.4172/2169-0022.1000258
Il’ina EA, Antonov BD, Vlasov MI (2020) Stability investigations of composite solid electrolytes based on Li7La3Zr2O12 in contact with LiCoO2. Solid State Ionics 356:115452. https://doi.org/10.1016/j.ssi.2020.115452
Il’ina EA, Raskovalov AA (2020) Studying of superionic solid electrolyte Li7La3Zr2O12 stability by means of chemical thermodynamics for application in all-solid-state batteries. Electrochim Acta 330:135220. https://doi.org/10.1016/j.electacta.2019.135220
Acknowledgements
The research has been carried out with the equipment of the Shared Access Center “Composition of Compounds” of the Institute of High-Temperature Electrochemistry of Ural Branch of RAS, Yekaterinburg, Russian Federation.
Funding
This work was funded by the Research Program № 122020100210-9 (IHTE UB RAS), Russian Academy of Sciences, Ural Branch, Russia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Il’ina, E.A., Lyalin, E.D., Kuznetsova, T.A. et al. Cathode modification by Li2O–B2O3–SiO2 glass addition for all-solid-state battery creation. Ionics 28, 3635–3642 (2022). https://doi.org/10.1007/s11581-022-04640-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04640-1