Abstract
Rice straw is made up of hemicelluloses (19–27%), celluloses (32–47%), lignin (5–24%), and ash (13–20%), which are all agricultural waste. Rice straw ash is considered a green/eco-friendly source of silicon dioxide (SiO2). This study focuses on the synthesis and characterization of different mesoporous SiO2 nanostructures derived from rice straw waste material through controlling the pH of the extraction process for the first time. X-ray diffraction (XRD), Fourier transform infrared (FTIR), diffuse reflectance spectroscopy (DRS), field emission scanning electron microscope (FESEM), energy dispersive X-ray spectroscopy (EDX), high-resolution transmission electron microscope (HRTEM), zeta potential, and surface area analyzer were used to examine the produced materials. Amorphous silica nanostructures, S3 and S7, were produced at pH values of 3 and 7, respectively, according to XRD measurement, whereas higher pH causes the production of crystalline silica (S9). The pH of the extraction has a major effect on the morphology of the resultant nanosilica, as S3 has an irregular shape, S7 is made of distorted spherical particles, and S9 is composed of mixed fiber and spherical particle structures. For pollutant removal, greenly produced SiO2 nanostructures were used. The optimal mesoporous nanosilica (S9) demonstrated the highest surface roughness, the largest surface area (262.1 m2/g), the most negative zeta potential (− 20.2 mV), and the best dye adsorption capacity (71.4 mg/g).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Millions of people throughout the world rely on rice as their primary source of nutrition, making it an increasingly important crop. Because of the rapid increase in population, there has been a recent surge in the demand for rice consumption all over the world [1, 2]. One of the most significant problems that arise from the cultivation of rice is an oversupply of rice straw that is not put to any use as a by-product. Unfortunately, the great majority of farmers dispose of rice straw in the paddy field by an illegal method known as open burning. This practice contributes to the pollution of the air and has a negative impact on human health due to the mobility of soot particles. Furthermore, the fact that farmers discard paddy straw in public areas where it increases the insect problem. Utilizing rice straw and converting trash into wealth are both fantastic ways to make effective use of agricultural by-products and materials that would otherwise be thrown away. Rice straw has cellulose content ranging from 30–45%, along with hemicellulose and lignin at 25–15%, ash at 13–20%, and a negligible amount of organic compounds like protein. Rice straw has an abundance of resources, but they have little commercial value, which makes it excellent for the manufacture of silica nanoparticles in large quantities. Studies done in the past have proven without a reasonable doubt that rice paddy straw is one of the key sources that contain significant amounts of silica minerals [3, 4]. Powdered silicon dioxide or silica has a lot of uses in industry and everyday life. It is extensively utilized either as a base material in numerous industries (cement, glass, porcelain, and refractory) or as a filler for rubber, tire, plastic, and so on [5]. SiO2 is originally a natural material present in the form of quartz sand. However, supplying silicon dioxide from natural minerals consumes a lot of energy and, if done frequently, can harm the ecosystem. Therefore, silicon dioxide made from plants is often used instead of silicon dioxide that comes from minerals [6]. Rice plants get silica from the soil by polymerizing water-soluble silicic acid (H4SiO4) into insoluble polysilicic acids, which are subsequently precipitated in the form of silica and implanted in plant cell walls. From wasted rice straw, around 10 × 106 tons of silica may be produced [7]. Researchers have detailed the successful manufacture of biosilica nanoparticles through efficient and straightforward method using rice straw. Ash paddy straw reacts with a strong base (sodium hydroxide), producing sodium silicate [Eq. (1)]. After that, the filtered sodium silicates were titrated with acid, Eq. (2), to reach the pH down to 7. As a result of this processes, silica is formed [4].
Khorsand et al. [8] presented the synthesis of silica from Iranian rice straw ash with a diameter of 10–15 nm. The process was carried out at various concentrations of sodium hydroxide. In another study, rice straw was subjected to enzymatic hydrolysis for effective decomposing biomass and then heat treatment step at 500 °C for synthesis of amorphous silica [9]. Materials related to SiO2 have attracted a lot of interest recently in the field of water purification, specifically in the processes of degradation and adsorption [10]. Quartz, cristobalite, and tridymite are three examples of the crystalline forms that can be found in silicon dioxide. In addition, the amorphous phase of silicon dioxide is the most important form of the material since it may be utilized in a number of fields. Crystalline quartz and amorphous silicon oxide both have optical qualities that have stronger transition strengths and indices of refraction than other materials. In the spectrum of the inter-band transition strength, crystalline silicon oxide exhibits more distinct features than its amorphous one [11, 12]. There is a lot of interest in making crystalline and amorphous materials for use in microelectronics, optics, electricity, and other fields. High-purity silica nanoparticles could be used in many areas, including water treatment, sensor devices, catalysis, and dielectric materials [13, 14]. By adsorption, silicon oxide nanoparticles have been used to get rid of pollutants like antibiotics and heavy metals [12]. Another interesting field is the removal of dyes from the wastewater of textile factories [15, 16]. It is becoming widely attractive to employ natural, biocompatible, bio-waste, and residues generated by the food, agricultural, and wood industries as more cost-effective and environmentally favorable sorbents. Arefieva et al. [17] removed methylene blue from aqueous solutions through adsorption on amorphous silicon dioxide made from rice husks. Amibo et al. [18] used biosilica from teff straw for the elimination of methyl orange dye. Nguyen et al. [19] prepared SiO2 NPs extracted from rice husk ash for adsorptive removal of iron. Manaa et al. [20] used SiO2/C derived from rice straw for the removal of chromium (VI).
Green chemistry deals with the use of strategies/technologies to eliminate or hinder the use/formation of harmful raw materials, products, by-products, solvents, reagents, etc., that are harmful to human health and/or the environment. Climate change has become a major concern in the modern world. The production of greenhouse gases is at its highest level ever, contributing to global warming [21,22,23,24]. Hence, the major goal of this study is to solve the problems associated with the rice straw waste as well as the synthesis of silicon dioxide that is critical in various fields. What’s more, several investigations have extracted SiO2 from rice straw, but the presented synthesis strategy produced different mesoporous SiO2 nanostructures with improved characteristics by changing the extraction conditions for the first time. In the literature, the nanoparticle is the common morphology for the formed SiO2, whereas the fiber and spherical mixed morphology of the prepared mesoporous nanosilica (S9) have not been investigated before. All the synthesized materials were well-characterized using XRD, FTIR, DRS, FESEM, EDX, HRTEM, zeta potential, and surface area. Finally, the obtained green nanosilica samples were utilized for dye removal and showed improved performance relative to the literature. The use of agro-waste-derived silica for the elimination of harmful pollutants is an excellent procedure to achieve two tasks in one step: promoting a safe route to cure agricultural effluents and, on the other side, proposing an alternative option to minimize the utilization of chemical-based adsorbents/photocatalysts for the removal of organic pollutants.
Materials and methods
Materials
Rice straw waste was collected from a rice paddy in Egypt. Analytical grade chemicals (HCl and NaOH) were obtained from Merck. Methylene blue was purchased from SD Fine Chemicals.
Synthesis of SiO2 nanostructures
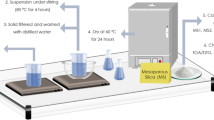
The sol–gel method was utilized for green synthesis of nanosilica from rice straw as a raw biomass. The rice straw was collected, washed several times with water, and then dried overnight at 70 °C. The clean rice straw was put into crucibles and burned for 4 h at 900 °C. In order to get sodium silicate, the ash was refluxed with 3 N NaOH at 85 °C for three hours. Then, HCl was added drop by drop to the sodium silicate solution until the pH reached 3, 7, and 9 to get three different samples of nanosilica, S3, S7, and S9, respectively. The precipitated nanosilica was centrifuged, washed with deionized water, and finally dried at 40 °C. Scheme 1 shows a flowchart for the synthesis of SiO2 nanostructures in addition to the morphology of the grown silica according to morphological analyses.
Dye adsorption experiments
Methylene blue (MB) was chosen as an example of organic pollutant. The adsorption tests were performed by adding 20 mg of the sample to 40 mL of dye solution under stirring at 25 °C. Every 30 min, 3 mL was taken out and centrifuged at 7000 rpm for 2 min. After that, Shimadzu 2401 PC UV–Vis spectrophotometer was used to measure the UV–visible absorption spectra of the collected filtrate, and then the remaining dye concentration was calculated. Every adsorption experiment was repeated twice, and the average value was reported.
The removal percentage (%R) and the adsorption quantity (qt) of the MB dye were estimated using Eqs. (3, 4), respectively.
where \(C_{0}\): initial dye concentration, \(C_{t}\): dye concentration at specific time, V: volume of MB solution (L), and m: mass of adsorbent (g). In order to identify the most efficient SiO2 sample, the adsorption performance of as-prepared SiO2 samples was evaluated in terms of removal efficiency and adsorption capacity. Using the optimum SiO2 sample, the effects of varying adsorption time (30, 60, 90, 120, 150, 180, 210, and 240 min), adsorbent dose (10, 20, 30, and 40 mg), and starting MB concentration (10, 15, 20, 25, 30, 35, and 40 mg/L) were investigated. To further understand the mechanics of the MB adsorption process onto the optimum SiO2, the data were fitted to various kinetic models (pseudo-first order, pseudo-second order, intraparticle diffusion, and Elovich) and isothermal models (Freundlich, Langmuir, Dubinin–Radushkevich, and Temkin).
Measurement techniques
On a Bruker VERTEX 80, an attenuated total reflection-Fourier transform infrared (ATR-FTIR) examination was carried out with a resolution of 4 cm−1 in the range 4000 –400 cm−1. In order to verify the crystal structure, X-ray diffraction (XRD) measurements were performed using a PANalytical X'Pert PRO diffractometer with a wavelength equivalent to 1.540 A° target Cu–K. The UV–Visible/NIR spectrophotometer (Jasco V-770) was used to measure the diffuse reflectance spectra (DRS) of the powders over the wavelength range of 190 − 1000 nm. Quanta 250 FEG was used in a field emission scanning electron microscope (FESEM) equipped with an EDX detector in order to examine the surface morphology and chemical composition of the SiO2 samples. The roughness features of the FESEM images were estimated with the use of the Gwyddion software, which generates 3D micrographs. In order to demonstrate the shape and particle size of the prepared SiO2, a high-resolution transmission electron microscope (HRTEM) was carried out using a JEM-2100F electron microscope equipped with a voltage accelerating source of 200 kV. A Quantachrome apparatus was utilized in order to calculate the Brunauer–Emmett–Teller (BET) specific surface area, and the DFT approach was utilized in order to obtain an approximation of the pore size distribution. Zeta potential was measured using a NICOMP 380-ZLS, USA.
Results and discussion
Characterization of silica nanostructures
FTIR spectra analysis
Figure 1 represents the FTIR spectra of the prepared nanosilica samples, including S3, S7, and S9, which were prepared at pH 3, 7, and 9, respectively. The weak band at ~ 1630 cm−1 is related to the bending H–O–H of adsorbed water molecules. The peak around 1050 cm−1–1070 cm−1 was assigned to the asymmetric stretching of siloxane groups (Si–O–Si). Band around 960 cm−1 − 940 cm−1 was related to the stretching vibration of silanol group (Si–OH). The symmetric stretching of Si–O–Si was observed at about 800 cm−1. The bending vibration of O–Si–O was appeared at 454 cm−1. It has been noticed that only S9 has a band at ~ 880 cm−1, which was attributed to the bending vibration of Si–OH [4, 25,26,27,28]. This may suggest that the S9 sample contains a higher content of silanol (Si–OH) groups that are effective for improving interaction with cationic MB dye in comparison with other silica samples, as can be seen in the adsorption experiments section. Finally, the FTIR spectrum of S9 shows a clear shift of Si–O–Si asymmetric and symmetric stretching peaks toward a lower wave number that reflects the smaller Si–O–Si bond angle. Angle reduction accounts for the increase in intrinsic stress. As a result, Si–O–Si groups become more reactive to H2O attack, and their strained bonds are preferentially broken to 2Si–OH [29]. Based on the above assignment, FTIR analysis confirmed the successful formation of SiO2 from rice straw.
XRD analysis
XRD analysis was performed to determine changes in the development of amorphous or crystalline silica prepared at different pH (3, 7, and 9). Figure 2 shows XRD patterns of all the prepared silicon dioxide. As seen in Fig. 2, S3 and S7 show amorphous peak at 2θ = 22.2°, which is assigned to amorphous SiO2 structure according to previous literature [5, 30]. There are no other peaks visible, indicating the high purity of the generated SiO2. For the XRD pattern of SiO2 prepared at pH = 9 (S9), the formed SiO2 is in crystalline form which was related to the SiO2 crystalline cristobalite phase, according to previous literature [18, 31, 32]. The peaks at about 31.7°, 45.4°, and 56.5° are attributed to (102), (202), and (104) lattice plane, respectively, according to (JCPDS 00-082-1403) [33]. The crystalline form of SiO2 improves the strength and stability of SiO2 in order to perform its functions properly.
DRS analysis
The optical properties of the formed silicon dioxide samples were investigated by measuring the diffuse reflectance spectra (DRS) in order to calculate the band gap energy using the Kubelka–Munk function FKM (R) according to the following equations [34, 35].
where Eg is the band gap energy and hν is the photon energy. Figure 3 depicts the relation between (FKM(R)hν)2 and E (eV). The estimated band gap energy is 5.47 eV for S3, 5.58 eV for S7, and 5.06 eV for S9. It is noticed that S9 has the lowest band gap energy compared to other samples. Generally, these values are near the band gap of the chemically synthesized SiO2 [36]. Many researches have demonstrated that SiO2 sensitization improves the photocatalytic activity of the obtained SiO2-based nanocomposites [37, 38]. For example, Kusdianto et al. [38] studied the photocatalytic activity of ZnO/SiO2 nanocomposite in comparison with pure ZnO under UV and solar irradiation. They found that under solar irradiation, the degradation efficiency of ZnO/SiO2 can reach 96% owing to the incorporation of SiO2. It is concluded that this research can open the way for further eco-friendly, green, and cost inexpensive SiO2-based nanocomposites to be developed in order to address the world's photocatalytic and wastewater treatment issues.
FESEM and EDX analysis
Surface morphology and chemical composition nanosilica samples (S3, S7, S9) that were prepared at different pH were studied using FESEM and EDX, respectively, as presented in Fig. 4. The SEM micrographs of S3 (Fig. 4a) and S7 (Fig. 4c) show irregular and dense particles like foam with a size ranged from 48 to 50 nm. On the other hand, S7 (Fig. 4e) consists of more dispersed particles relative to other samples, in addition to the fiber-like structure with a diameter in the range of 70–300 nm. We must point out that although there are several articles on the production of nanosilica using chemical or green methods, the majority of them form SiO2 has spherical or semi-spherical morphology [4, 5, 18, 37], none have investigated nanosilica with fiber/particle mixed morphology. EDX analysis spectra (Fig. 4b, d, f) showed Si and O elements, indicating the formation of SiO2. Na, Cl, C, and K exist in low/negligible amounts.
Figure 5 represents 3D images of SiO2 prepared at different pH. An essential property for activating series interaction in a variety of applications, including drug delivery, antibacterial agency, and water filtration, is the material's ability to adhere to its surroundings. As a result, compositional tailoring is used to regulate roughness manipulation and activity [39]. The root mean square roughness and average roughness are important parameters that are used to describe the surface roughness. As a result, the physical adhesion of the materials formed is greatly affected by these characteristics. Also, roughness heights serve as hooks that facilitate interaction between the sample and its surroundings. As seen in Table 1, S9 has the highest root mean square roughness, average roughness, and maximum peak height. This could lead to an increase in the amount of dye that can be absorbed because of the increased active sites.
HRTEM analysis
Figure 6 shows HRTEM images of nanosilica produced at various pH values. As seen in Fig. 6a, S3 exhibited varied forms that coagulated into large clusters of approximately 45 nm in size, with grain boundaries that were not clearly defined. For S7, Fig. 6c, the particles have irregular spherical like shape with average particle size of 9 nm and are agglomerated with each other. For S9, Fig. 6e, the particles have spherical shape with defined grain boundaries, low coagulation, and average particle size of 6 nm. Also, S9 has a rod-like shape as a second morphology with an approximate diameter of 11 nm and this agrees with SEM analysis. This conclusion was consistent with the findings of earlier studies, which demonstrated that the pH value had a significant impact on the dimensions, shape, and characteristics of nanomaterials [40]. The influence of extraction pH on the morphology of the obtained silica is clearly visible in the results, which demonstrate that the particle size decreases as the pH increases.
Surface area analysis
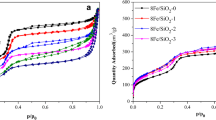
In particular, the material's surface area is a critical feature that influences its adsorption ability. Figure 7 shows the nitrogen adsorption–desorption isotherm and the corresponding pore size distribution of all SiO2 nanostructures. It's interesting to note that all SiO2 samples displayed type-IV adsorption–desorption isotherms with H3 type hysteresis loop at P/P0 > 0.5, indicating the mesoporous character [41,42,43]. Additionally, pore size distribution curves in the inset of Fig. 7 show that all SiO2 samples were mesoporous structures (porous in the range of 2–50 nm). Table 2 displays the values of specific surface area, average pore size, and total pore volume. The surface area is 120.3 m2/g for S3, 121.3 m2/g for S7, and 262.1 m2/g for S9. It is noticed that all the prepared SiO2 nanostructures in this study have higher surface area than the SiO2 derived from rice husk, which has only 98.2 m2/g surface area, as reported by Vu et al. [44]. Recently, Singh et al. [31] presented that the surface area of crystalline nanosilica, which was prepared from rice straw, was 84.45 m2/g. Sankar et al. [45] prepared spherical SiO2 nanoparticles from brown rice husk with a surface area in the range of 201.45−271.2 m2/g. In addition, SiO2 with 20.98 m2/g surface area was prepared from bamboo leaf ash [46]. In another study, SiO2 nanoparticles were produced from sugarcane waste ash with surface area of 131 m2/g [47]. The total pore volume of S3, S7, and S9 is 0.450, 0.490, and 1.093 cc/g, respectively. The average pore size is 7.48, 8.10, and 8.34 nm for S3, S7, and S9, respectively. Based on these results, we concluded that at pH 3 and 7, as-synthesized SiO2 samples have similar textural parameters, including specific surface area, total pore volume, and average pore size. On the other hand, S9, prepared at pH 9, possesses more than double the specific surface area relative to other samples. In addition, the same sample (S9) exhibited the largest pore size and pore volume. This can provide more adsorption sites and led to a positive effect on the adsorption capability of the prepared S9 toward organic pollutants.
Zeta potential analysis
The zeta potential values of as-synthesized SiO2 nanostructures, including S3, S7, and S9, are listed in Table 2. It is seen that all samples develop a negative charge, which may be due to the hydroxyl groups on their surface. SiO2 prepared at lower pH (pH = 3) has the lowest negative zeta potential of − 12.66 mV, whereas increasing the pH of the SiO2 synthesis conditions increased the zeta potential up to − 19.53 mV and then, after a further increase in pH to 9, the value of the zeta potential increased slightly to − 20.2 mV. This may be attributed to that the silanol groups (Si–OH) in silicon dioxide are deprotonated at high alkaline synthesis conditions and transformed to Si–O–, consequently the silica surface becomes more negative [48]. According to these, it is predicted that S9 demonstrates stronger electrostatic attraction toward positively charged contaminants, such as cationic MB dye, as can be seen in the dye adsorption experiments section.
Dye adsorption studies
Effect of synthesis conditions on the adsorption activity of SiO2 nanostructures
The MB uptake by as-prepared SiO2 samples (S3, S7, and S9) was investigated as presented in Fig. 8. Based on these UV–Vis spectra, the major peak of MB at 664 nm exhibited a significant decline in the following order S9 > S7 > S3. This trend is proportional to the increase in the pH used for the synthesis of SiO2. Figure 8c shows the calculated MB removal percentage for all samples at 180 min. The removal efficiency was 62.05% for S3, 75% for S7, and 95.1% for S9. It is noticed that SiO2 sample prepared at higher pH, S9, revealed the highest dye decorlorization in comparison with other samples. This may be attributed to its higher OH groups, as presented in FTIR data, which can effectively interact with the cationic MB dye and lead to more dye decolorization. In addition, S9 acquires more negative zeta potential and higher surface area (as shown in the characterization section) that are beneficial for enhancing adsorption characteristics. In conclusion, S9 prepared at higher alkaline conditions exhibited the best adsorption abilities, so it was selected as the optimum sample in other experiments.
Effect of initial dye concentration
The adsorption performance experiments of the most efficient Si2O sample (S9) were conducted using MB with different initial concentrations, including 10, 15, 20, 25, 30, 35, and 40 mg/L, and then the corresponding %R and qt were calculated as shown in Fig. 9a, b, respectively. The adsorbent dosage was 20 mg, and the adsorption time was 240 min. As depicted in Fig. 9a, there is an inverse relationship between the starting MB concentration and the dye removal percentage. This performance is attributed to that as the dye concentration rises, but the active adsorption sites on the adsorbent material are constant, hence increasing the initial dye concentration reduces decolorization efficiency. Similar findings were found in other research [49, 50]. The impact of initial concentration of MB on the adsorption capacity of S9 was also evaluated as seen in Fig. 9b. It was found that the adsorption capability of the S9 increased significantly with rising initial MB concentration from 10 to 30 mg/L. After that, the absorption capacity tends to remain unchanged at higher starting concentrations (C0 > 35) owing to the saturation of all adsorption points in the material. Similar findings were obtained for the elimination of MB using γ-Fe2O3/SiO2 [51].
Effect of adsorbent dose
Based on the high adsorption properties of S9, the experiments to examine the optimal adsorbent dose were investigated. The test conditions were C0 = 20 mg/L and time = 240 min. Figure 10 shows the effect of changing the S9 dosage from 10 to 40 mg on the MB removal efficiency and adsorption amount. The results revealed that MB removal significantly increased from 76.5 to 95.1% with increasing dose from 10 to 20 mg. However, a further increase in dose (> 20 mg) did not cause any noticeable variation in MB decolorization. It was revealed that adding greater doses of the adsorbent material increases the number of active sorption positions; as a result, the decolorization efficiency increases up to a certain point and then remains almost constant when the amount of adsorbent material is raised further [52]. The same trend was observed in the case of adsorption capacity, where at 20 mg dose, the S9 adsorption capacity increased up to 38.1 mg/g and then maintained almost constant. As a result, an adsorbent dosage of 20 mg was the optimum choice for further research.
Effect of adsorption time
Figure 11 indicates that the percentage of MB dye removed by the most efficient SiO2 sample (S9) increases rapidly in the first 30 min and then progressively increases over time until it reaches an almost constant value. The fast adsorption rate demonstrated at the beginning was most likely due to the abundance of active sites on the surface of SiO2 particles, while the steady increase in the latter stage was probably owing to the lack of adsorption sites and diffusion of the organic dye molecules into the interior of the adsorbent material [53].
Adsorption kinetic studies
40 mL of MB solution with starting concentration of 20 mg/L and 20 mg of the optimal SiO2 sample (S9) were used in the adsorption kinetic tests. The pseudo-first order (PFO), pseudo-second order (PSO), interparticle diffusion (IPD), and Elovich models were used to match the experimental data for MB sorption onto the S9 sample under nonequilibrium conditions. The experimental results are well-matched to the kinetic model as the R2 value (correlation coefficient) approaches unity. The values of kinetic model parameters and R2 are summarized in Table 3.
The equation of the pseudo-first-order (PFO) model is presented as follows [54].
where qe: the adsorption quantity in mg/g at equilibrium, qt: the adsorption quantity at specific time t (min), and K1: the pseudo-first-order rate constant (min−1). ln(qe–qt) vs time is shown in Fig. 12a. The values of K1 and qe were calculated using the slope and intercept of this curve, respectively. Table 3 compares experimental and estimated qe by this model and also the values for correlation coefficient (R2) and K1. It is obvious that the R2 value is low, and the computed adsorption capacity differs from the experimental one. The results demonstrated that MB adsorption on S9 is not a PFO process.
The pseudo-second-order (PSO) model is as follows [55]:
where K2: the PSO rate constant. As illustrated in Fig. 12b t/qt is plotted against time. The slope and intercept were used to determine the values of K2 and qe, as shown in Table 3. It is observed that R2 = 0.999, as well as excellent agreement between the calculated and measured qe. These findings demonstrate that MB adsorption onto S9 is a PSO process in which the chemisorption step controls the rate. Also, the adsorption kinetic results for MB adsorption onto γ-Fe2O3/SiO2 nanocomposite were consistent with a PSO model [51].
Equation (9) represents the intraparticle diffusion (IPD) model [56].
The IPD constant is Ki, and the boundary layer thickness is C. It was reported that when C is equal to zero, the intraparticle diffusion is the determining step. As illustrated in Fig. 12c, qt is plotted against t1/2. The lower R2 value (0.943) and also the fact that the straight line can't pass through the origin, led to the conclusion that intraparticle diffusion isn’t the suitable model to describe the MB adsorption.
The Elovich model is given by Eq. (10) [57].
where β: the desorption constant and α: the initial adsorption rate. In Fig. 12d, qt is plotted against ln(t). The obtained R2 value (0.974) revealed that the Elovich model didn’t well match the MB adsorption process.
Adsorption isotherm models
To describe MB dye adsorption on the surface of the optimum SiO2 (S9) under equilibrium conditions, the experimental results are fitted to several sorption isotherm models, including Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich. The best-fitted isotherm model is chosen based on the correlation coefficient (R2) value for these models. Table 4 shows the R2 and parameters for each model.
Langmuir isotherm model suggests the formation of uniform monolayer coverage owing to that adsorption sites have the same ability to the adsorbate. Equation (11) is Langmuir's linear form [58].
where qmax: the calculated maximum adsorption quantity and KL: the Langmiur constant. Ce/qe is drawn against Ce, as illustrated in Fig. 13a. The values of qmax and KL are determined from the slope and intercept. In addition, using Eq. (12), the isotherm model type is defined based on the value of the separation factor (RL). The adsorption can be favorable, unfavorable, reversible, and irreversible at 0 < RL < 1, RL > 1, RL = 1, and RL = 0, respectively [59]. The Langmuir model is the best fit model because of its high R2 (0.995). Furthermore, at C0 = 20 mg/L, the value of RL is 0.027, indicating that MB adsorption onto SiO2 (S9) is favorable.
The Freundlich isotherm model is given by Eq. (13). This model assumes that the adsorbate molecules (MB) cover the adsorbent surface (S9) in several layers [60].
where KF: the Freundlich constant, and the adsorption intensity is n. At 1/n = 1, 1/n < 1, and 1/n > 1, the adsorption is homogeneous, favorable, and unfavorable, respectively [61,62,63]. Figure 13b shows the plot of lnqe versus lnCe, where slope = 1/n and intercept = lnKF. From the data in Table 4 1/n is greater than 1, indicating the favorable adsorption of MB dye. However, the low R2 value (0.892), indicates that the MB dye adsorption on the SiO2 does not follow the Freundlich isotherm model.
Temkin isotherm postulates that the heat of adsorption decreases owing to the interaction between adsorbent and adsorbate (dye), and its equation is as follows [64,65,66]:
where bT: Temkin isotherm constant, A: equilibrium binding constant, R: universal gas constant, and the temperature (K) is T [62]. As shown in Fig. 13c, qe is plotted against lnCe. The R2 of the Temkin model is 0.918, showing that it isn’t the suitable model to describe the MB adsorption onto SiO2.
Equation (15) shows the Dubinin–Radushkevich isotherm that considers the adsorption on both homogeneous and heterogeneous surfaces [57, 67].
where qD-R: the adsorption capacity calculated by this model, β: the mean adsorption free energy, the Polanyi adsorption is ε. Equations (16, 17) are used to compute the Polanyi adsorption and average free energy (E), respectively [68]. ε2 is plotted against lnqe as shown in Fig. 13d. Based on the value of E, the sorption process is defined as a physical, ion exchange, chemisorption process at E < 8, 8 < E < 16, and 16 < E < 40, respectively [69]. From the results in Table 4, E = 2.02 kJ/mol, the MB adsorption on the surface of SiO2 is a physical process. The Dubinin–Radushkevich isotherm didn’t well match the MB adsorption, as evidenced by the relatively low value of R2 (0.983). The same results were observed by Chen et al. [51] for MB adsorption on the surface of γ-Fe2O3/SiO2.
Comparative study
The effectiveness of any adsorbent can be demonstrated by comparing its adsorption capacity to that of other adsorbents reported in the literature. The adsorption capabilities of several adsorbents studied for MB adsorption are summarized in Table 5. Group A represents examples of natural adsorbent materials. When compared to different natural adsorbents in group A, such as banana peel [70, 71], orange peel [71], lemon peel [72], yellow passion fruit peel [73], fly ash [74], natural zeolite [75], rice husk [76, 77], and rice straw [78], for treating MB in wastewater, the adsorption capacity of the produced SiO2 nanostructure (71.4 mg/g) is much higher. There are various trials to enhance the adsorption efficiency of natural materials via different modifications, as mentioned in group B. For example, Fathy et al. [78] found that the adsorption capacity of rice straw was increased from 32.6 to 62.9 mg/g after alkali treatment. EDTA modification for rice husk was reported by Ong and co-workers [79]. They showed that the EDTA-treated rice husk (ERH) exhibited higher adsorption affinity for cationic MB relative to natural rice husk (NRH). The adsorption capacity of ERH reached 46.30 mg/g. Finally, the adsorption capabilities of several silica and silica-based adsorbents are listed in group C for comparison. Recently, Motawea et al. [48] prepared ZrO2/rice straw-derived SiO2 with 13.5 mg/g adsorption capacity for MB dye. Two-dimensional mesoporous SiO2 can be prepared using petal cells as a biotemplate and tetraethyl orthosilicate as a silica source [80]. They found that MB adsorption capacity of 2D SiO2 (74 mg/g) is significantly greater than that of nonporous SiO2 (14 mg/g) and traditional mesoporous SBA-15 (45 mg/g) that was owing to the characteristic two-dimensional porous structure. Dhmees and co-authors [81] prepared nanosilica from commercial blast furnace slag for the elimination of MB dye. The adsorption capacity is 80.8 mg/g in basic conditions. In conclusion, this comparison indicates that the produced mesoporous SiO2 with fiber/particle mixed morphology in this study has greater or comparable adsorption properties, that it might be used as an effective adsorbent material for eliminating toxic contaminants existing in wastewater.
Reusability
Economic considerations highlight the importance of material reusability in adsorption procedures. In a study of SiO2 nanostructure (S9) regeneration, washing of MB-adsorbed S9 with ethanol resulted in simple desorption. After drying S9, it was used in the second cycle and so on. Four regenerations of S9 have been performed to examine its reusability. The experimental results, as depicted in Fig. 14, showed that the MB removal percentage using a regenerated S9 sample was nearly identical to that of S9 in its original state. Slight decrease in the removal capability of the S9 nanosilica may be due to MB molecules that remained on its surface that most likely were chemisorbed onto nanosilica. Even after four rounds of recycling, the removal rate remains above 93%. This indicates that the green synthesized nanosilica has good stability, which will increase the catalysts' lifespan and reduce costs in practical uses.
Adsorption mechanism
To elucidate the interactions between adsorbent material and dye molecules, several possible adsorption processes have been postulated, including electrostatic interaction, ion exchange, hydrogen bonding, acid–base, and coordination interaction [85]. Based on zeta potential values of as-prepared SiO2 nanostructures, the surface of all adsorbents is negatively charged and the MB molecules, in fact, are positively charged. Therefore, cationic MB dye can be adsorbed through electrostatic interaction onto the surface of all SiO2 samples to some extent. In addition, the MB removal efficiency is enhanced as the surface charge of SiO2 becomes more negative, so S9 exhibited higher adsorption properties relative to other samples (S3 and S7). Recently, Singh et al. [68] mentioned that the existence of Si–O and Si–OH groups on the silica surface gives a negative charge, making silicon dioxide a suitable substrate for adsorbent cationic dyes. Also, Subhan et al. [86] presented that MB adsorption onto Fe3O4@SiO2 occurred due to electrostatic attraction between the Si–O– of silica and MB cations. What’s more, hydrogen bonds can be formed between silanol groups (Si–OH) of silica and nitrogen of cationic dye [42, 85, 87]. As a result, electrostatic attraction and hydrogen bonding could be used to elucidate MB adsorption on the surface of silica, as shown in Fig. 15.
Conclusion
Rice straw is natural, non-toxic, eco-friendly, low-cost, and a vital raw material for the production of high-quality silica. According to the analyses and results, changing the pH of the silicon dioxide production process has a significant effect on the nanosilica's properties. FTIR and EDX measurements, regardless of pH, confirmed the production of SiO2. The XRD analysis indicated that amorphous silica was produced under acidic and neutral conditions, while crystalline silica was produced under basic conditions. Morphological analysis revealed that S3 and S7 were grown in the form of particles. In contrast, the S9 sample exhibits fiber-like features in addition to less agglomerated and more uniform spherical particles. Studies on dye adsorption demonstrated that S9 displayed superior dye adsorption capacity compared to S3 and S7. The improved performance observed can be explained by the zeta potential and surface area measurements. At pH = 3, nanosilica exhibited the least negative zeta potential (− 12.66 mV); nevertheless, increasing the pH to 7 increased the zeta potential to − 19.53 mV, and then a further rise in pH to 9 slightly increased the zeta potential value to − 20.2 mV. According to these findings, S3 demonstrated the least MB removal efficiency. Additionally, the surface area of S9 was double that of other silica samples (S3 and S7). Therefore, the synergistic combination of a greater negative zeta potential and a large surface area increased the dye adsorption characteristics of S9 nanosilica.
References
Uda MNA, Shaari NH, SaidNS IbrahimNH, Akhir MA, Hashim MKR, Salimi MN, Nuradibah MA, Hashim U, Gopinath SC (2018) Antimicrobial activity of plant extracts from aloe vera, citrus hystrix, sabah snake grass and zingiber officinale against pyricularia oryzae that causes rice blast disease in paddy plants. IOP Conf Ser Mater Sci Eng 318(1):012009
Uda MNA, Gopinath SC, Hasfalina CM, Faridah S, Bunawan SN, Sabrina WN, Parmin NA, Hashim U, Afnan Uda MN, Mazidah M (2018) Production and purification of antibody by immunizing rabbit with rice tungro bacilliform and rice tungro spherical viruses. Process Biochem 68:37–42
Yasina M, Bhuttob AW, Bazmia AA, Karimb S (2010) Efficient utilization of rice-wheat straw to produce value–added composite products. Int J Chem Environ Eng 1(2):136–143
Uda MNA, Gopinath SC, Hashim U, Halim NH, Parmin NA, Afnan Uda MN, Anbu P (2021) Production and characterization of silica nanoparticles from fly ash: conversion of agro-waste into resource. Prep Biochem Biotechnol 51(1):86–95
Deshmukh P, Bhatt J, Peshwe D, Pathak S (2012) Determination of silica activity index and XRD, SEM and EDS studies of amorphous SiO2 extracted from rice husk ash. Trans Indian Inst Met 65(1):63–70
Har NP, Irzaman I (2019) Crystallinity and electrical properties of silicon dioxide (SiO2) from rice straw. AIP Conf Proc 2202:1–6
Sarkar J, Mridha D, Sarkar J, Orasugh JT, Gangopadhyay B, Chattopadhyay D, Roychowdhury T, Acharya K (2021) Synthesis of nanosilica from agricultural wastes and its multifaceted applications: a review. Biocatal Agric Biotechnol 37:102175
Khorsand H, Kiayee N, Masoomparast AH (2013) Optimization of amorphous silica nanoparticles synthesis from rice straw ash using design of experiments technique. Part Sci Technol 31(4):366–371
Wattanasiriwech S, Wattanasiriwech D, Svasti J (2010) Production of amorphous silica nanoparticles from rice straw with microbial hydrolysis pretreatment. J Non-Cryst Solids 356(25–27):1228–1232
Batool SS, Imran Z, Hassan S, Rasool K, Ahmad M, Rafiq MA (2016) Enhanced adsorptive removal of toxic dyes using SiO2 nanofibers. Solid State Sci 55:13–20
Lenza RF, Vasconcelos WL (2001) Preparation of silica by sol-gel method using formamide. Mater Res 4(3):189–194
Ali AA, Nassar MY, Shama SA, El Sharkwy AM, El Sayed NE (2020) Sol-gel auto-combustion synthesis and identification of silicon dioxide nanoparticles for the removal of sunset dye from aqueous solutions. Benha J Appl Sci 5(7):217–229
Dubey RS, Rajesh YBRD, More MA (2015) Synthesis and characterization of SiO2 nanoparticles via sol-gel method for industrial applications. Mater Today Proc 2(4–5):3575–3579
Nandanwar R, Singh P, Haque FZ (2015) Synthesis and characterization of SiO2 nanoparticles by sol-gel process and its degradation of methylene blue. Am Chem Sci J 5(1):1–10
Kale RD, Kane PB (2017) Colour removal using nanoparticles. Text Cloth Sustain 2(1):1–7
Wang Z, Yu C, Fang C, Mallavarapu M (2014) Dye removal using iron–polyphenol complex nanoparticles synthesized by plant leaves. Environ Technol Innov 1:29–34
Arefieva OD, Zemnukhova LA, Gorlova VA, Tsvetnov MA (2021) Removal of methylene blue from aqueous solutions by adsorption on amorphous silicon dioxide from rice husks. Water Pract Technol 16(2):351–363
Amibo TA, Beyan SM, Damite TM (2022) production and optimization of bio-based silica nanoparticle from teff straw (eragrostis tef) using rsm-based modeling, characterization aspects, and adsorption efficacy of methyl orange dye. Journal of Chemistry
Nguyen TT, Ma HT, Avti P, Bashir MJ, Ng CA, Wong LY, Jun HK, Nago QM, Tran NQ (2019) Adsorptive removal of Iron using SiO2 nanoparticles extracted from rice husk ash. Journal of analytical methods in chemistry
Manaa AE (2015) Extraction of highly pure silica from local rice straw and activation on the of the left carbon for chromium (VI) adsorption. Chem Technol Indian J 10(6):242–251
Abel S, Tesfaye J, Gudata L, Nagaprasad N, Subramanian K, Mani M, Shanmugam R, Dwarampudi LP, Roy A, Stalin B Krishnaraj R (2022) Biobutanol preparation through sugar-rich biomass by clostridium saccharoperbutylacetonicum conversion using ZnO nanoparticle catalyst. Biomass Conversion and Biorefinery 1–11.
Saka A, Jule LT, Gudata L, Shuma S, Nagaprasad N, Subramanian K, Afessa G Ramaswamy K (2022) Preparation of biobutanol via coffee bean harsh extracts by zinc oxide nanoparticle as catalyst. Biomass Conversion and Biorefinery 1–10
Saka A, Enkosa E, Jule LT, Nagaprasad N, Subramanian K, Ramaswamy K (2022) Biofuel production from mango (mangifera indica) seed extracts through zinc oxide nanoparticle. Biomass Conversion and Biorefinery 1–11
Saka A, Gudata L, Jule LT, Seeivasan V, Nagaprasad N, Ramaswamy K (2022) Synthesis of nano-sized lead sulfide thin films from avocado (glycosmis cochinchinensis) leaf extracts to empower pollution remediation. Sci Rep 12:1–9
Nandiyanto ABD, Rahman T, Fadhlulloh MA, Abdullah AG, Hamidah I, Mulyanti B (2016) Synthesis of silica particles from rice straw waste using a simple extraction method. IOP Conf Ser Mater Sci Eng 128(1):012040
Oh T, Choi CK (2010) Comparison between SiOC thin films fabricated by using plasma enhance chemical vapor deposition and SiO2 thin films by using fourier transform infrared spectroscopy. J Korean phys soc 56(4):1150–1155
Grumezescu AM, Ghitulica CD, Voicu G, Huang KS, Yang CH, Ficai A, Vasile BS, Grumezescu V, Bleaotue C, Chifiriuc MC (2014) New silica nanostructure for the improved delivery of topical antibiotics used in the treatment of staphylococcal cutaneous infections. Int J Pharm 463(2):170–176
Martínez JR, Espericueta D, Guerrero-Serrano G, Ortega-Zarzosa G, Espericueta E, Guerrero AL (2020) Stabilization of β-carotene embedded in a silica matrix and study of its physical properties. Mater Res Express 7(1):015205
Scherer K, Nouvelot L, Lacan P, Bosmans R (1996) Optical and mechanical characterization of evaporated SiO2 layers. Long-term evol Appl opt 35(25):5067–5072
Liang Y, Ouyang J, Wang H, Wang W, Chui P, Sun K (2012) Synthesis and characterization of core–shell structured SiO2@ YVO4: Yb3+, Er3+ microspheres. Appl Surf Sci 258(8):3689–3694
Singh G, Dizaji HB, Puttuswamy H, Sharma S (2022) Biogenic nanosilica synthesis employing agro-waste rice straw and its application study in photocatalytic degradation of cationic dye. Sustainability 14(1):539
Nazopatul PH, Irmansyah Irzaman (2018) Extraction and characterization of silicon dioxide from rice straw. IOP Conf Ser Earth Environ Sci 209:012013
Jiang Y, Jiang FQ, Liao X, Lai SL, Wang SB, Xiong XQ, Zheng J, Liu YG (2020) Customized three-dimensional porous catalyst for knoevenagel reaction. J Porous Mater 27(3):779–788
Ali H, Ismail N, Mekewi M, Hengazy AC (2015) Facile one-step process for synthesis of vertically aligned cobalt oxide doped TiO2 nanotube arrays for solar energy conversion. J Solid State Electrochem 19(10):3019–3026
Mbakaan C, Ahemen I, Amah AN, Onojah AD, Koao L (2018) White-light-emitting Dy3+-doped amorphous SiO2 nanophosphors derived from rice husk. Appl Phys A 124(11):1–9
Abdel Messih MF, Shalan AE, Sanad MF, Ahmed MA (2019) Facile approach to prepare ZnO@ SiO2 nanomaterials for photocatalytic degradation of some organic pollutant models. J Mater Sci Mater Electron 30(15):14291–14299
Babu LK, Rao HS, Kishore PNR, Reddy YR (2019) Hydrothermal synthesis of flower-like ZnO–SiO2 nanocomposites for solar light–induced photocatalysis and anti-bacterial applications. Materials Research Express 6(8): 08504
Kusdianto K, Widiyastuti W, Shimada M, Qomariyah L, Winardi S (2020) Fabrication of ZnO–SiO2 nanocomposite materials prepared by a spray pyrolysis for the photocatalytic activity under UV and sunlight irradiations. IOP Conf Ser Mater Sci Eng 778(1):012105
Al-Ahmed ZA, Al-Radadi NS, Ahmed MK, Shoueir K, El-Kemary M (2020) Dye removal, antibacterial properties, and morphological behavior of hydroxyapatite doped with Pd ions. Arab J Chem 13(12):8626–8637
Thi TUD, Nguyen TT, Thi YD, Thi KHT, Phan BT, Pham KN (2020) Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv 10(40):23899–23907
Zheng CH, Zhang GP, Wang SS, Mao AQ, Fang DL (2021) Efficient transformation of rice husk to a high-performance Si@ SiO2@ C anode material by a mechanical milling and molten salt coactivated magnesiothermic reduction. J Alloy Compd 875:159974
Qin P, Yang Y, Zhang X, Niu J, Yang H, Tian S, Zhu J, Lu M (2017) Highly efficient, rapid, and simultaneous removal of cationic dyes from aqueous solution using monodispersed mesoporous silica nanoparticles as the adsorbent. Nanomaterials 8(1):4
Li Y, Zhou Y, Nie W, Song L, Chen P (2015) Highly efficient methylene blue dyes removal from aqueous systems by chitosan coated magnetic mesoporous silica nanoparticles. J Porous Mater 22(5):1383–1392
Vu AT, Xuan TN, Lee CH (2019) Preparation of mesoporous Fe2O3·SiO2 composite from rice husk as an efficient heterogeneous fenton-like catalyst for degradation of organic dyes. J Water Process Eng 28:169–180
Sankar S, Kaur N, Lee S, Kim DY (2018) Rapid sonochemical synthesis of spherical silica nanoparticles derived from brown rice husk. Ceram Int 44(7):8720–8724
Fatimah I, Prakoso NI, Sahroni I, Musawwa MM, Sim YL, Kooli F, Muraza O (2019) Physicochemical characteristics and photocatalytic performance of TiO2/SiO2 catalyst synthesized using biogenic silica from bamboo leaves. Heliyon 5(11):e02766
Rovani S, Santos JJ, Corio P, Fungaro DA (2018) Highly pure silica nanoparticles with high adsorption capacity obtained from sugarcane waste ash. ACS Omega 3(3):2618–2627
Motawea MM, Hussein MA, Elsenety MM, Ali HM, El-Nasr TAS, Gomaa H (2022) Mesoporous hierarchical ZrO2@ rice straw-derived SiO2 nanocomposite for rapid adsorption and sunlight-driven photocatalytic degradation of methylene blue. J Photochem Photobiol A 426:113758
Heybet EN, Ugraskan V, Isik B, Yazici O (2021) Adsorption of methylene blue dye on sodium alginate/polypyrrole nanotube composites. Int J Biol Macromol 193:88–99
Ali H, Ismail AM (2021) Structural, morphological, and enhanced dye sorption properties of mesoporous TiO2/ZnS nanocomposite synthesized by SILAR method. J Inorg Organomet Polym Mater 32(1):326–343
Chen D, Zeng Z, Zeng Y, Zhang F, Wang M (2016) Removal of methylene blue and mechanism on magnetic γ-Fe2O3/SiO2 nanocomposite from aqueous solution. Water Resour Ind 15:1–13
Liu Q, Yang B, Zhang L, Huang R (2015) Adsorption of an anionic azo dye by cross-linked chitosan/bentonite composite. Int J Biol Macromol 72:1129–1135
Ali H, Tiama TM, Ismail AM (2021) New and efficient NiO/chitosan/polyvinyl alcohol nanocomposites as antibacterial and dye adsorptive films. Int J Biol Macromol 186:278–288
Lagrergen S (1898) Zur theorie der sogenannten adsorption gelöster stoffe kungliga svenska vetenskapsakademiens. Handlingar 24(4):1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–59
Ghibate R, Senhaji O, Taouil R (2021) Kinetic and thermodynamic approaches on Rhodamine B adsorption onto pomegranate peel. Case Stud Chemical Environ Eng 3:100078
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Khawaja H, Zahir E, Asghar MA, Asghar MA (2021) Graphene oxide decorated with cellulose and copper nanoparticle as an efficient adsorbent for the removal of malachite green. Int J Biol Macromol 167:23–34
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57(1):385–470
Jain P, Sahoo K, Mahiya L, Ojha H, Trivedi H, Parmar AS, Kumar M (2021) Color removal from model dye effluent using PVA-GA hydrogel beads. J Environ Manage 281:111797
Muhammad A, Shah AUHA, Bilal S, Rahman G (2019) Basic blue dye adsorption from water using polyaniline/magnetite (Fe3O4) composites: kinetic and thermodynamic aspects. Materials 12(11):1764
Mahmoodi NM, Hayati B, Arami M, Bahrami H (2011) Preparation, characterization and dye adsorption properties of biocompatible composite (alginate/titania nanoparticle). Desalination 275(1–3):93–101
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta physiochim URSS 12:327–356
Paluri P, Ahmad KA, Durbha KS (2020) Importance of estimation of optimum isotherm model parameters for adsorption of methylene blue onto biomass derived activated carbons: comparison between linear and non-linear methods. Biomass Conversion and Biorefinery 1-18
Liu Y, Liu YJ (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61(3):229–242
Dubinin MM (1947) The equation of the characteristic curve of activated charcoal. Dokl Akad Nauk SSSR 55:327–329
Singh S, Perween S, Ranjan A (2021) Dramatic enhancement in adsorption of congo red dye in polymer-nanoparticle composite of polyaniline-zinc titanate. J Environ Chem Eng 9(3):105149
Ali H, Ismail AM (2021) Developing montmorillonite/PVDF/PEO microporous membranes for removal of malachite green: adsorption, isotherms, and kinetics. J Polym Res 28(11):1–17
Amela K, Hassen MA, Kerroum D (2012) Isotherm and kinetics study of biosorption of cationic dye onto banana peel. Energy Procedia 19:286–295
Annadurai G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater 92(3):263–274
Kumar KV, Porkodi K (2006) Relation between some two-and three-parameter isotherm models for the sorption of methylene blue onto lemon peel. J Hazard Mater 138(3):633–635
Pavan FA, Lima EC, Dias SL, Mazzocato AC (2008) Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J Hazard Mater 150(3):703–712
Kumar KV, Ramamurthi V, Sivanesan S (2005) Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J Colloid Interface Sci 284(1):14–21
Reddy KR, Lee KP, Gopalan AI, Kang HD (2007) Organosilane modified magnetite nanoparticles/poly (aniline-co-o/m-aminobenzenesulfonic acid) composites: synthesis and characterization. React Funct Polym 67(10):943–954
Zhang YP, Lee SH, Reddy KR, Gopalan AI, Lee KP (2007) Synthesis and characterization of core-shell SiO2 nanoparticles/poly (3-aminophenylboronic acid) composites. J Appl Polym Sci 104(4):2743–2750
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci 286(1):90–100
Fathy NA, El-Shafey OI, Khalil LB (2013) Effectiveness of alkali-acid treatment in enhancement the adsorption capacity for rice straw: the removal of methylene blue dye. International Scholarly Research Notices
Ong ST, Lee WN, Keng PS, Hung YT, Ha ST (2010) Equilibrium studies and kinetics mechanism for the removal of basic and reactive dyes in both single and binary systems using EDTA modified rice husk. Int J Phys Sci 5(5):582
Cui R, Lin Y, Qian J, Zhu Y, Xu N, Chen F, Liu C, Wu Z, Chen Z, Zhou X (2017) Two-dimensional porous SiO2 nanostructures derived from renewable petal cells with enhanced adsorption efficiency for removal of hazardous dye. ACS Sustain Chem Eng 5(4):3478–3487
Dhmees AS, Khaleel NM, Mahmoud SA (2018) Synthesis of silica nanoparticles from blast furnace slag as cost-effective adsorbent for efficient azo-dye removal. Egypt J Pet 27(4):1113–1121
Salimi F, Tahmasobi K, Karami C, Jahangiri A (2017) Preparation of modified nano-SiO2 by bismuth and iron as a novel remover of methylene blue from water solution. J Mex Chem Soc 61(3):250–259
Oppmann M, Wozar M, Reichstein J, Mandel K (2019) Reusable superparamagnetic raspberry-like supraparticle adsorbers as instant cleaning agents for ultrafast dye removal from water. ChemNanoMat 5(2):230–240
Han H, Wei W, Jiang Z, Lu J, Zhu J, Xie J (2016) Removal of cationic dyes from aqueous solution by adsorption onto hydrophobic/hydrophilic silica aerogel. Colloids Surf A 509:539–549
Yuan N, Cai H, Liu T, Huang Q, Zhang X (2019) Adsorptive removal of methylene blue from aqueous solution using coal fly ash-derived mesoporous silica material. Adsorpt Sci Technol 37(3–4):333–348
Subhan F, Aslam S, Yan Z, Khan M, Etim UJ, Naeem M (2019) Effective adsorptive performance of Fe3O4@ SiO2 core shell spheres for methylene blue: kinetics, isotherm and mechanism. J Porous Mater 26(5):1465–1474
Belkassa K, Khelifa M, Batonneau-Gener I, Marouf-Khelifa K, Khelifa A (2021) Understanding of the mechanism of crystal violet adsorption on modified halloysite: hydrophobicity, performance, and interaction. J Hazard Mater 415:125656
Acknowledgements
Dr. Ali expresses gratitude to Prof. Zahra Saleh of the NRC's Centers of Excellence and Central Laboratories Network for optical measurements.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds were received.
Author information
Authors and Affiliations
Contributions
AS Conceptualization, Formal analysis, Methodology, Writing-original draft; AMI Data curation, Formal analysis, Software, Writing-original draft; HA Data curation, Formal analysis, Software, Visualization, Writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Chris Cornelius.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samy, A., Ismail, A.M. & Ali, H. Environmentally friendly mesoporous SiO2 with mixed fiber/particle morphology and large surface area for enhanced dye adsorption. J Mater Sci 58, 1586–1607 (2023). https://doi.org/10.1007/s10853-022-08119-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-08119-2