Abstract
Magnesium alloy has excellent physical and mechanical properties and is expected to become a new generation of medical degradable materials. In this paper, the blood compatibility analysis of Graphene Oxide(GO)/Hydroxyapatite(HA)-AZ91D was carried out to improve the blood compatibility of magnesium alloy materials. AZ91D was used as the control group and GO/HA-AZ91D was used as the experimental group. The two samples were studied in vitro by hemolysis rate test, cell proliferation test, NO release and T-AOC test. The results combined with the work of the research group showed that the blood compatibility and cell compatibility of the GO/HA-AZ91D group were significantly improved, providing an effective idea for future corrosion resistant coatings on magnesium alloys.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnesium alloy has excellent comprehensive mechanical properties and can be degradable and absorbed in the body, which is expected to become a new generation of medical implants. So, it has become a research hotspot in the field of degradable materials due to its excellent physical and mechanical properties [1, 2]. So far, many studies have been reported on magnesium alloys in the fields of orthopedics and cardiovascular stents [3,4,5]. Ji [6] has previously studied Graphene Oxide/Hydroxyapatite-AZ91D Magnesium alloy (GO/HA-AZ91D), and the corrosion resistance of the composite coating is significantly improved. According to the definition of International Standards Organization (ISO), blood compatibility [7] refers to the required reaction of blood to exogenous substances or materials, and generally refers to the compatibility between materials and components in blood. The main visible components of blood are blood cells, including red, white and platelets, which play an important role in maintaining nutrient and oxygen supply, inflammation and clotting, respectively.
Hemolysis, platelet adhesion and activation caused by biomaterials are the main contents of blood compatibility research. After GO/HA-AZ91D is implanted into the human body, blood clots may occur when the blood flows through, causing great harm. In order to reduce the formation of thrombosis, the blood compatibility of materials is particularly important. The evaluation and detection method of blood compatibility of biological materials mainly refer to the international standard ISO10993-4.
GO/HA coating can improve the surface properties of materials to a certain extent and enhance the blood compatibility of materials. In this paper, GO/HA-AZ91D was analyzed for blood compatibility in order to improve the blood compatibility of magnesium alloy materials.
Experimental materials and methods
Experimental materials
AZ91D, HA-AZ91D and GO/HA-AZ91D are made by our lab. Human umbilical vein endothelial cells (HUVECs) were purchased by Shanghai Meixuan Biological science and technology LTD. Normal Blood was collected from the eight adult male Wistar rats (200 ± 20 g, about two months), which were purchased from Laboratory animal center (The Second Affiliated Hospital of Harbin Medical University, China). The animals had free access to a commercial pellet diet and drinking water before all experiments. We do not abuse animals and any adverse effects were noted during the experiments.
All procedures were performed in this experiment had previously been approved by the School of Materials Science and Chemical Engineering, Harbin University of Science and Technology and all efforts were made to minimize suffering and the number of rats used.
Hemolysis rate test
When biomaterials are implanted into the body, they may react with red blood cells, causing them to rupture or release hemoglobin from the red blood cells through partial damage. Blood compatibility is an important evaluation index to evaluate whether the biocompatibility of biomaterials is appropriate. Hemolysis test is one of the tests to test the blood compatibility of materials and is used to measure the degree of RBC lysis and hemoglobin release caused by biomaterials in vitro. According to the biological evaluation standard of medical devices, when the hemolysis rate is greater than 5%, it indicates that the biomaterial has hemolysis phenomenon, which cannot meet the requirements of medical materials. When the hemolysis rate is less than 5%, it meets the requirements of medical materials for hemolysis rate.
In this experiment, AZ91D, HA-AZ91D and GO/HA-AZ91D samples were taken, respectively, 10 mL normal saline was added into the test tube, and preheated at 37°C for 30 min. The sample and 0.2 mL diluted blood (blood: normal saline = 1:5) were placed in a test tube and placed in a CO2 incubator at 37°C for 60 min. Normal saline as negative control, distilled water (three steamed water) as positive control. All test tubes were centrifuged at 3000 RPM /min for 5 min. The supernatant was taken and the absorbance of the supernatant was measured at wavelength 545 nm with distilled water as reference.
(Dt-absorbance of test samples; Dnc-absorbance of negative control; Dpc-absorbance of positive control).
If the hemolysis rate is less than 5%, it indicates that the material meets the requirements of hemolysis test for medical materials. If the hemolysis rate is greater than 5%, it shows that the test material has certain hemolysis effect.
Preparation of the extract
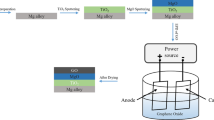
Magnesium alloy is easy to corrode, and can be evaluated by using extracts when evaluating cellular compatibility. After the material is completely treated, it is placed under ultraviolet light overnight to sterilize. RPMI1640 medium containing 10% serum was used for preparation. The ratio of the sample surface area to the extraction medium was 1.25 cm2/mL and placed at a temperature containing 5% carbon dioxide. It was carried out in a CO2 incubator at 37 °C for 72 h. The extract was prepared with RPMI1640 medium at 25%, 50% and 100% concentrations and placed in a 4 °C refrigerator for later use.
Determination of magnesium ion in the extract
The concentration of magnesium ion in the extract affected the proliferation and adhesion of cells. Magnesium test kit was used in this experiment to test the content of magnesium ions in different groups. Normal RPMI 1640 medium containing 10% serum was used as control.
Cell culture
HUVECs were used in this paper Use the 10% serum RPMI1640 medium was cultured in an incubator containing 5% carbon dioxide at 37 °C.
Cell proliferation assay
In the presence of electron carrier 1-Methoxy PMS, WST-8 is reduced by dehydrogenase in cells to a highly water-soluble yellow methylzan product. The amount of methylzan produced is proportional to the number of living cells. This property can be used directly for cell proliferation and toxicity analysis. WST-8 is a compound similar to MTT that can be reduced by some dehydrogenases within the mitochondria to produce orange-yellow Formazan in the presence of an electron-coupled reagent. If the cells proliferate faster, the color will be darker; if the cytotoxicity is greater, the color is lighter. For the same cell, there is a linear relationship between the shade of color and the number of cells.
In this experiment, CCK-8 kit was used to detect the cell proliferation and cytotoxicity of coated magnesium alloy. The specific steps of this experiment are as follows:
-
①
Cell suspension of 1 × 104 cells/mL was prepared.
-
②
Inoculated 100 μL of cell suspension into 96-well plates, 3 replicates were performed for each sample, and normal cells were used as negative control. The 96-well plate was placed in a CO2 incubator for 24 h. A 96-well plate was taken out at day 1, day 3 and day 5.
-
③
After cell adherent culture, the original medium was replaced with extracts of different concentrations. 10 μL CCK-8 kit was added to each well at 1st, 3rd and 5th day detection time points. About 2 h later, the absorbance of each well was measured at 450 nm with immunomicroplate analyzer. And the mean value of the three repeated Wells was calculated.
The relative cell proliferation rate was calculated by the following formula.\( \begin{aligned} & {\text{Relative}}\;{\text{Growth}}\;{\text{Rate}}\left( {{\text{RGR}},\% } \right) = {\text{Average}}\;{\text{absorbance}}\;{\text{value}}\;{\text{of}}\;{\text{experimental}}\;{\text{group}} \\ & \quad {\text{/average}}\;{\text{absorbance}}\;{\text{value}}\;{\text{of}}\;{\text{negative}}\;{\text{control}}\;{\text{group}}*100\% \\ \end{aligned} \)
Results were then graded according to toxicity criteria specified in national safety standards [8] shown in Table 1.
Nitric oxide (NO) determination
Different concentrations of extracts were still used in this experiment. Cell suspension with a concentration of 4 × 104 cells/mL was prepared, and each well was inoculated with 500 μL in 24-well plates, and 3 cells were repeated.
The normal cultured cells were used as the control group. After the cells adhered to the wall, the original culture was replaced with different concentrations of extracts.
The supernatant of cells was taken after 24 h of culture. The subsequent experimental steps were carried out according to the NO kit instructions. The absorbance of each well was measured at 570 nm with an immunomicroplate reader. The content of NO is proportional to the absorbance, and the higher the absorbance, the higher the content of NO. Absorbance was used to compare the content of NO in each group of cells.
Total antioxidant capacity (T-AOC) determination
The antioxidant capacity of the body's defense system is closely related to health. This defense system includes two parts: enzymatic and non-enzymatic reactions, for example, SOD, CAT, GSH—PX, VC, VE, GSH, β-carotene, etc. Their role is to decompose free radicals, remove catalytic metal ions, when the body's total antioxidant capacity is reduced, it is easy to cause inflammation, cancer, diabetes, immune system and other diseases. First, cell suspension with a cell concentration of 2 × 104 cells/mL was prepared, and 1 mL of fine cell suspension was inoculated into 24-well plates with 3 replicates per well. The subsequent experimental steps were carried out in strict accordance with the total antioxidant energy kit. Finally, the absorbance of each well was tested at 520 nm in UV–vis spectrophotometer. The unit content of total antioxidant capacity was still positively correlated with absorbance, and the difference of total antioxidant capacity of each group was still compared according to the absorbance.
Experimental results
Hemolysis rate test results
The surface properties of GO/HA-AZ91D have changed greatly. As shown in the Table 2, severe hemolysis occurred in AZ91D group, and the hemolysis rate was as high as 15.22%. HA-AZ91D group was 8.36%. However, GO/HA-AZ91D has a hemolysis rate of 4.88%, lower than 5%, showing good blood compatibility and meeting the requirements of medical materials.
The content of magnesium ion in the extract
The content of magnesium ion has a great influence on tissue cells. The corrosion rate of AZ91D is so fast that the magnesium ion content in local tissues was too high after the material was implanted in the body, which causes changes in body function. The composite coating can effectively slow down the corrosion rate of magnesium alloy and reduce the concentration of magnesium ion. The extraction method was the same as before, and RPMI1640 medium was used as the control group. After 72 h, the experimental results showed that there was a significant difference in magnesium ion content between AZ91D and GO/HA-AZ91D. As shown in Fig. 1, the magnesium ion content in AZ91D group was as high as 27.9 ± 2.3 mmol/L, while the magnesium ion content after GO/HA coating was no different from that in normal medium. These results indicate that GO/HA-AZ91D coating is indeed beneficial to slow down the corrosion rate of magnesium alloys.
Cell proliferation and toxicity results
CCk-8 was used to compare the effect of magnesium alloy material on cell proliferation before and after coating. The results of cell proliferation after 1 day of culture are shown in Fig. 2. Compared with the normal cell group, both the AZ91D group and the GO/HA-AZ91D group have faster relative proliferation rates than the normal cells. However, 100% AZ91D extract, 50% and 100% GO/HA-AZ91D had a greater effect on the proliferation rate of cells, and there was no significant difference between other groups and normal cells.
Cell proliferation results after 3 days of culture are shown in Fig. 3. Two concentrations in AZ91D group had a great impact on cell proliferation, while one concentration in GO/HA-AZ91D had an impact on cell proliferation.
Cell proliferation results after 5 days of culture are shown in Fig. 4. AZ91D at 100% concentration had an impact on cell proliferation, and there was no significant difference between other concentration groups and normal cells.
The results showed that the high magnesium ion content in the extract promoted the cell proliferation at the initial stage of culture, however, with the extension of time, the high magnesium ion content in the extract inhibited the cell proliferation. The high concentration extract of AZ91D only promoted the effect on the first day, while it showed strong inhibition on the third and fifth day of culture. GO/HA-AZ91D has a strong effect on promoting cell proliferation on the first day of cell culture, and there is no difference between the cell proliferation results and the control group at 3 and 5 days of culture.
The cytotoxicity test results are shown in Table 3. In all the groups, the cytotoxicity grade was 0 after 1 day of culture. After 3 days of culture, AZ91D had two concentrations of level 2 cytotoxicity and GO/HA-AZ91D had only one level 1 cytotoxicity. After 5 days of culture, the cytotoxicity level of AZ91D was level 1 or level 2, and there were two concentrations of GO/HA-AZ91D, both of which were level 1, and only one concentration was level 0. In summary, GO/HA-AZ91D may promote cell proliferation due to the modification of the polymer.
Results of NO content
Angiogenesis refers to the formation of vascular lumen after proliferation and migration of endothelial cells on the basis of the original vascular network. Vascular endothelial cells are directly related to angiogenesis, and nitric oxide (NO) is one of the important regulatory signals of angiogenesis. The absorbance value of NO is shown in Fig. 5, and there was NO difference between each group. The reason may be that there is not significant relationship between NO signaling pathway and magnesium ion content. Each group showed better NO release than the normal group. This indicates that the NO release amount of magnesium alloy is appropriate.
T-AOC determination results
Reactive oxygen species (ROS) can be produced during normal physiological metabolism of biological tissues, and some environmental factors such as UV irradiation, γ ray irradiation, environmental pollution and other factors can also induce ros production. When reactive oxygen species are produced, they can lead to Oxidative damage of intracellular lipids, proteins and DNA, and induce Oxidative stress, which can then lead to various tumors, atherosclerosis, rheumatoid arthritis, diabetes, liver damage, and central nervous system diseases, etc. Therefore, it is particularly necessary to measure the total antioxidant capacity of cells. The results of total antioxidant capacity are shown in Fig. 6 In general, the overall antioxidant capacity of magnesium alloy before and after surface coating is significantly different from that of the normal group, and the total antioxidant capacity is significantly enhanced, indicating that magnesium alloy material has a good enhancement effect on the total oxidation capacity of cells.
Conclusions
After magnesium alloy is implanted into human body, biocompatibility is a complex chain process, blood compatibility and cellular compatibility are two basic contents of evaluation. Particularly for magnesium alloys, the high corrosion rate may have a great influence on their biocompatibility.
In this study, GO-HA was coated on the surface of magnesium alloy as a corrosion resistant polymer protective layer, and the hemolysis rate of magnesium alloy material before and after coating was detected. The hemolysis rate of coated magnesium alloy material was lower than 5%, meeting the requirements of medical materials. However, the hemolysis rate of AZ91D was as high as 15.22%, resulting in severe hemolysis. The reason for serious hemolysis may be the high content of AZ91D ions. When red blood cells contact with materials, red blood cells may produce different osmotic pressures on both sides of the cell wall, resulting in hemolysis. Therefore, we can know that the adverse hemolysis of magnesium alloy materials can be avoided by improving the corrosion resistance of magnesium alloy materials through appropriate surface modification. The hemolysis rate test proved that the coated magnesium alloy showed good blood compatibility.
Cytocompatibility was studied by cell proliferation assay, cytotoxicity assay, determination of NO content and T-AOC content. The effects of different concentrations of AZ91D and GO/HA-AZ91D extracts on the proliferation of human umbilical vein endothelial cells were detected using CCK-8 kit. The results showed that different concentrations of extracts had different effects on endothelial cells. For AZ91D, the higher the concentration, the greater the endothelial damage. However, the effect of GO/HA protection on endothelial cell proliferation in the experimental group was not as obvious as that in the control group. Especially AZ91D, the high concentration of the extract inhibited the proliferation of endothelial cells. Cytotoxicity experiments showed that AZ91D extracts at different concentrations had certain damage compared with the experimental group at 1, 3 and 5 days of cell culture, and GO/HA-AZ91D may be protected by GO/HA, resulting in less toxicity. Cell proliferation was detected by CCK-8 kit, and PHB, as a protective layer, promoted cell proliferation to a certain extent. The content of NO was not affected before and after coating, and there was NO difference compared with the normal group. However, the total antioxidant capacity of magnesium alloy before and after coating was greatly improved. In general, the coated magnesium alloy material has been improved in blood compatibility and cell compatibility to a certain extent, which lays a certain foundation for the future surface modification of magnesium alloy.
References
Espiritu J, Meier M, Seitz JM (2021) The current performance of biodegradable magnesium-based implants in magnetic resonance imaging: a review. Bioact Mater 6(12):4360–4367
Sekar P, Narendranath S, Desai V (2021) Recent progress in in vivo studies and clinical applications of magnesium based biodegradable implants—a review. J Magnes Alloys 9(4):1147–1163
Peng HZ, Fan K, Zan R (2021) Degradable magnesium implants inhibit gallbladder cancer. Acta Biomater 128:514–522
Estrin YUZ, Kiselevsky MV, Anisimova NYu et al (2019) Biodegradable magnesium alloys as promising materials for medical applications. Sovremennye tekhnologii v meditsine 11(3):146–157
Han HS, Loffredo S, Jun I (2019) Current status and outlook on the clinical translation of biodegradable metals. Mater Today 23:57–71
Ji HR, Li XW, Song YB et al (2020) Effect of fine-grained AZ91D coating with graphene oxide/hydroxyapatite on biocompatibility. ChemistrySelect 5:7768–7774
Ju MJ, Xu BB, Xu LG (2020) Synthesis and properties of blood compatible polyurethane elastomer. Chin. J. Org. Chem. 40(12):4344–4349
Farsalinos K, Lagoumintzis G (2019) Toxicity classification of e-cigarette flavouring compounds based on European Union regulation: analysis of findings from a recent study. Harm Reduct J 16:48–55
Funding
This study was funded by the Foundation of Heilongjiang Natural Science Project. (E2017051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ji, H., Zhao, Y., Yu, X. et al. Effect of Graphene Oxide/Hydroxyapatite fine-grained AZ91D on blood compatibility and cytocompatibility. J Mater Sci 57, 15008–15015 (2022). https://doi.org/10.1007/s10853-022-07565-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07565-2