Abstract
The morphology evolution by thermal annealing induced dewetting of gold (Au) thin films on silicon (Si) substrates with a native oxide layer and its dependences on annealing temperature and atmosphere are investigated. Both dewetting degree of thin film and Au/Si interdiffusion extent are enhanced with the annealing temperature. Au/Si interdiffusion can be observed beyond 800 °C and Au–Si droplets form in both argon and oxygen (Ar + O2) and argon and hydrogen (Ar + H2) environments. In Ar + O2 case, the passive oxidation (Si + O2 → SiO2) of diffused Si happens and thick silicon oxide (SiOx) covering layers are formed. A high temperature of 1050 °C can even activate the outward growth of free-standing SiOx nanowires from droplets. Similarly, annealing at 800 °C under Ar + H2 situation also enables the slight Si passive oxidation, resulting in the formation of stripe-like SiOx areas. However, higher temperatures of 950–1050 °C in Ar + H2 environment initiate both the SiOx decomposition and the Si active oxidation (2Si + O2 → 2SiO(g)), and the formation of solid SiOx is absent, leading to the only formation of isolated Au–Si droplets at elevated temperatures and droplets evolve to particles presenting two contrasts due to the Au/Si phase separation upon cooling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
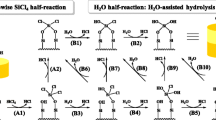
As the building blocks of modern integrated circuits, the interactions of gold (Au) thin film and silicon (Si) substrate have attracted extensive attention [1,2,3,4,5]. Au–Si droplets can be easily formed due to the low eutectic temperature (363 °C) based on Au–Si phase diagram [6, 7]. In the past decade, the Au–Si droplets have been commonly used as catalysts to grow Si or silicon oxide (SiOx) nanowires (NWs) based on the solid–liquid-solid (SLS) or vapor–liquid-solid (VLS) mechanisms [8,9,10,11,12,13]. The main difference between the two mechanisms is the type of the Si source that plays a crucial role in the fabrication of NWs and their morphologies. Previously, we demonstrated that the thickness of the silicon dioxide (SiO2) layer between Au thin film and Si substrate is critical for the involvement of three Si sources (diffused Si, SiO from decomposition of SiO2, and SiO from oxidation of Si) and the different morphologies of the evolved nanostructures [14].
The solid Si source is usually provided by the diffusion from the Si substrate at high temperatures [10, 13]. However, such Si diffusion will become more hindered as the SiO2 interlayer between film and substrate is thicker. In the presence of a reducing atmosphere (Ar + H2) at high annealing temperatures, the decomposition reaction of the SiO2 layer (Si + SiO2 → 2SiO(g)) happens [5, 15, 16], and its product, volatile SiO, can act as a vapor Si source for the formation of NWs [14, 17, 18]. Once the SiO2 layer is decomposed, the exposed Si substrate can be actively oxidized (2Si + O2 → 2SiO(g)) and this active oxidation reaction can also produce volatile SiO for the VLS-grown NWs [14, 17, 19, 20]. The SiO2 decomposition can be clearly observed in the case of a thick SiO2 layer: The formation of many circular and dark cavities on the surface [14,15,16,17, 21, 22]. Unfortunately, the contribution of the SiO2 decomposition to the synthesis of NWs was less discussed in the case of Si substrate with a native SiO2 layer [18], and researchers often considered it to be negligible because the native oxide interlayer was too thin at around 3 nm, and the Si source is only attributed to Si active oxidation [14, 19, 20]. However, more and more evidence show that the situation with native oxide is also more complex than that expected. Thus, it is significantly necessary to unravel influencing factors in the Au/Si system with a native oxide interlayer.
In the present work, Au/Si system with a native oxide interlayer is thermally annealed at temperatures 500–1050 °C in two annealing atmospheres (Ar + H2 and Ar + O2) for a short period of 1 min, respectively. At low temperatures of 500 °C and 650 °C, the Si diffusion is weak in both atmospheres, and no clear differences are found after the short-term annealing compared with previous results [23,24,25,26]. The Au/Si interdiffusion is enhanced and formed Au–Si droplets sink into Si substrate as the annealing temperature further increases. The Si passive oxidation (Si + O2 → SiO2) on droplets occurs for samples annealed at temperatures 800–1050 °C in Ar + O2 atmosphere as well as the sample treated at 800 °C in Ar + H2 atmosphere. By contrast, the initiation of SiO2 decomposition and Si active oxidation (2Si + O2 → 2SiO(g)) at higher temperatures in Ar + H2 atmosphere avoids the formation of solid oxidation products and only leads to the formation of Au–Si droplets that evolve to isolated particles showing bright and dark parts upon cooling. The morphologies and compositions of obtained structures were characterized, and different schematics were proposed to help the better understanding of Si oxidation behaviors.
Materials and methods
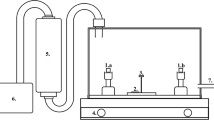
A thin Au film of thickness 20 nm was deposited onto the p-type Si (100) substrates by electron beam evaporation (CS400ES, VON ARDENNE) at a base pressure of 2 × 10–7 mbar. The thickness of Au film was determined by the quartz crystal monitor. Prior to deposition, Si substrates were cleaned with acetone, isopropanol, and deionized water and dried with N2. Thus, there was only a thin native SiO2 layer of 3 nm between Si substrate and Au film [14]. A fresh sample was thermally treated at each of the different temperatures from 500 to 1050 °C for 1 min by using a rapid thermal processing (RTP, Jipelec Jetstar 100) furnace. Fig. S1 shows the recorded temperature profile for each isothermal treatment. Gas flow containing Ar + H2 or Ar + O2 (volume ratio Ar:H2/O2 ~ 30:1) was applied to uncover the influences of the annealing atmosphere. Detailed annealing steps could be checked in our previous work [17]. The sample is labeled by its annealing temperature and atmosphere. For example, sample heated at 500 °C for 1 min in Ar + H2 environment will be named 500-H, while sample heated at 500 °C for 1 min in Ar + O2 environment will be referred to as 500-O.
The formed microstructures were investigated by high-resolution scanning electron microscopy (SEM, Hitachi S-4800), equipped with a detector for energy-dispersive X-ray spectroscopy (EDS, Thermo Scientific). The SEM images were taken at a voltage of 5 kV, while both secondary electron (SE) and backscattered electron (BSE) images were jointly used to gather topographic and compositional information: Areas containing higher atomic number elements will show brighter contrast. EDS measurements were carried out to determine the composition of the target microstructures and they were performed at 7 kV. Transmission electron microscopy (TEM, probe Cs corrected JEM-ARM300F2 cold FEG with JEOL dual-SDD EDS detector (2 × 158 mm2)) was performed at 300 kV to carefully check the morphology and composition of interested areas in the samples of 800-H and 1050-O. TEM lamella preparations were performed in a focused ion beam (FIB, ZEISS Auriga 60 CrossBeam) workstation equipped with Kleindiek MM3AEM micromanipulators. A layer of photoresist was spin-coated to stabilize the free-standing NWs in 1050-O, and several nanometers of chromium film were sputtered on top of the photoresist to increase the conductivity for the following operation in FIB. Before the cutting with FIB, a total of four protective layers, carbon and platinum layers deposited via electron beam and carbon and platinum layers deposited by Gallium ion beam, was deposited to protect the selected motifs. The height distribution was measured by an atomic force microscope (AFM, Bruker Dimension).
Results
Annealing in Ar + H2 atmosphere
An overview of samples heated in the reducing atmosphere is given in Fig. 1. SEM images show different dewetting stages upon annealing temperatures. A few voids are initiated in 500-H and branched void growth can be observed in 650-H. Similar dewetting behavior was found during the annealing of Au-Ni bilayers [23]. Material retraction is sped up at 800 °C, thus void impingement and further coalescence lead to the formation of isolated and elongated structures with some of them showing bright and dark parts. Moreover, stripe-like areas (indicated with dot circles in Fig. 1c) connecting those isolated structures can also be observed in 800-H. At 950 °C, isolated particles consisting of bright and dark parts can also be observed but above stripe-like areas are absent, and nearly every particle is located inside a circular and dark region. The obtained structures after heating at 1050 °C are similar to those formed at 950 °C with the main difference in particle size, and the circular regions around particles are hardly found in 1050-H. Figure 1f shows the EDS results of one particle in 800-H, and the element distributions along the arrow line of the inset indicate that bright parts of the particle are rich in Au while the dark part is rich in Si, which is also consistent with the material contrasts: Au shows brighter contrast than Si.
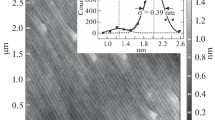
Figure 1c shows that the stripe-like areas always present the nearby of the isolated particles. The surface region near the particle is checked by TEM, and the cross-section view of the TEM lamella in Fig. S2 indicates that those isolated particles sink into the substrate in varying degrees. One particle is randomly chosen and its element distribution is given in Fig. 2a. The Au is mainly located in the isolated particle, suggesting the formation of liquid Au–Si droplets during the heating process. The oxygen segregates in the particle region and the near-surface region, meaning that the native oxide layer in 800-H still exists and the additional SiOx layer form around the particle. Thus, the stripe-like areas are supposed to be SiOx. The AFM height distribution profile in Fig. 2b indicates that both sides of the circular region are below the surface of the substrate, showing the concave feature, and the next these circular and dark regions will be referred to as cavities.
Annealing in Ar + O2 atmosphere
Another overview of samples heated in the oxidation atmosphere is given in Fig. 3, and values of degree of dewetting (dewetted area/total area) between two atmospheres are presented in Fig. S3. Similarly, small voids and branched void growth can be observed in 500-O and 650-O, respectively. Compared with 650-H, the voids in 650-O exhibit more branched morphology, and a higher degree of dewetting can also be found there, which can be explained by different surface energy anisotropy of Au thin films. The elevated rims near the voids exhibiting a terraced morphology will be formed by the material diffusion during the void expansion process, and the height of the agglomerated film keeps increasing till the end of the dewetting [27]. The higher degree of surface energy anisotropy for samples annealed in the reducing atmosphere, compared with that in the oxidation atmosphere, indicates the higher fraction of atomically flat facets on the edge surface of dewetted voids, resulting in the low effective diffusivity of Au atoms and the less branched features since the diffusion on atomically flat facets is quite difficult [28]. At 800 and 950 °C, the faster speeds of dewetting also lead to the formation of isolated and elongated structures with bright and dark parts. However, as indicated in Fig. S3, the degrees of dewetting in 800-O and 950-O are lower than their counterparts in reducing atmosphere. The possible reason for such opposite behavior with respect to a change in temperature attributes to the existence of a covering layer, as shown in the enlarged images in Fig. S4. The further reasons behind this are the initiation of Si diffusion at 800 °C and the passive oxidation of diffused Si, which will be discussed below. The height of the remained film increases by the material retraction as the dewetting processes. However, the formation of the covering layer prevents the thickening of the remained film and, therefore, slows down the dewetting rate in 800-O and 950-O. The dewetting is much more complete upon annealing at 1050 °C, and there are some free-standing NWs growing out from particles in 1050-O. The EDS results in Fig. 3f also confirm that the dark parts of isolated structures in 800-O are rich in Si.
The TEM results of 1050-O are shown in Fig. 4. Dispersed debris of free-standing NWs is encapsulated in the photoresist layer, as shown in Fig. 4a. Isolated particles sink into the Si substrate, and the covering layer around the particles can also be clearly observed. The covering layer presents different contrasts with the particles, indicating the possible difference in their compositions. Figure 4b gives the element mapping of a small area in Fig. 4a, and it shows that the Au is still mainly located in the particle. Si and O elements segregate at the location of dispersed debris, meaning that those free-standing NWs are SiOx. Also, Si and O show higher concentrations corresponding to the covering layer around the particle, which demonstrates that the covering layer is SiOx. Similar SiOx surface islands on top of the particles were also reported after the annealing of Au film/Si substrate sample in the Ar atmosphere [29].
Discussion
The above results clearly show that the temperature and ambient do affect the structure evolution during the annealing of Au film deposited on the Si substrate with a native SiO2 interlayer. However, the stripe-like structures observed in 800-H and their absence in 950-H as well as the structural differences between two atmospheres are less discussed. At 500 °C and 650 °C, the structure evolution seems to be negligibly affected by the atmosphere and the annealed samples show the initiation of solid-state dewetting with voids and the further branched void growth, and those processes have been clearly discussed [23, 26, 27]. Next, several schematics, as shown in Fig. 5, are proposed to deduce the evolution processes and help better understanding the obtained structures at temperatures 800–1050 °C.
Schematic diagrams of high-temperature structures at 800–1050 °C. a As-deposited sample, b Structure evolution showing the formation of stripe-like areas in 800-H, c Cavities around the particles in 950-H and the surface diffusion in 1050-H, d Samples treated in the Ar + O2 environment show the formation of the covering layer and even the growth of NWs in 1050-O. Si passive oxidation exists in b and d, while SiOx decomposition and Si active oxidation exist in c. The color evolution of particles indicates the higher and higher Si concentrations in Au–Si droplets as the temperature increases
Compared with the bright and dark parts shown in the isolated particles of 800-H, there are no special features in 500-H and 650-H, and the EDS results in Fig. 1f show that the dark parts are rich in Si. All these results indicate that the Si diffusion effectively happens at 800 °C in the reducing atmosphere. Besides, the results in Fig. S2 and Fig. 2a show that the particle sinks into the substrate, which means the Au atoms also diffuse into the substrate [14]. Therefore, the formation of stripe-like areas in Fig. 1c should be related to such Au/Si interdiffusion. As shown in Fig. 5b, the Au–Si droplets form at elevated temperature and the diffused Si can be slightly oxidized to SiOx shell around the droplet. As the further dewetting of the droplet, the elongated structures break into smaller particles due to the Rayleigh instability [27]. Those particles also sink into the substrate due to continuous Au/Si interdiffusion, then the previous SiOx shell breaks down. The processes in Fig. 5b circulate constantly, forming the stripe-like areas connecting many isolated particles.
The stripe-like structures are absent while the cavities around particles appear in 950-H. Although the etching of Au nanoparticles on SiO2 layer has been reported [19, 20], our results indicated that the formation of cavities could also be attributed to the SiO2 decomposition due to the appropriate annealing parameters (high temperature and O2-deficient atmosphere) [14, 17], and the circular feature of those cavities further prove the existence of the SiO2 decomposition [5, 14,15,16,17, 22]. However, the native SiO2 layer (around 3 nm) can only consume about 1.6 nm thickness of Si based on the calculation [17], and it cannot result in the concave areas with a depth of several tens nanometers. Accordingly, the active oxidation of Si should also happen within the dark regions where the native SiO2 layer is decomposed and the Si substrate exposes [14, 17, 19, 20]. Due to the small flow of H2, the probability of effective consumption of residual O2 molecules by H2 is still somehow limited, and this is confirmed by the O2 partial pressure calculation (supplementary materials). As marked in Fig. S5, the active oxidation of Si occurs in 950-H while passive oxidation of Si happens in 800-H because the calculated value of O2 partial pressure is slightly lower than the critical value at 950 °C but much larger than the critical value at 800 °C. Therefore, the existence of Si passive oxidation enables the formation of above stripe-like SiOx structures in 800-H, while Si active oxidation in 950-H indicates the formation of volatile oxidation product (SiO). In addition, the existence of SiO2 decomposition can consume SiOx that may be formed during the initial heating period. Finally, only isolated Au–Si droplets form at 950 °C and no stripe-like structures can be observed in 950-H. A similar SiO2 decomposition and Si active oxidation should also happen at a higher temperature of 1050 °C. Besides, the much bigger size of particles in 1050-H than that of 900-H, as shown in Fig. 1d, e indicate the initiation of Ostwald ripening among those isolated particles, and the strong surface diffusion of Au and Si is expected at 1050 °C [30]. The decomposition of SiO2 layer can be enhanced by the Au [14, 17, 22, 31], thus, the decomposition areas in 1050-H present a random distribution due to the strong surface diffusion and no clearly decomposed feature (circular areas) can be observed, as shown in Fig. 1e and the cross section in previous results [14]. By contrast, the weak surface diffusion at 950 °C enables the Au rich around particles and the SiO2 decomposition is locally enhanced, resulting in the typically circular decomposed areas in the vicinity of particles. Together with the further Si active oxidation within the decomposed areas, the clear cavities can be observed around the particles. The schematics of their high-temperature phases are given in Fig. 5c. Afterward, Au/Si phase separation happens during the cooling period and the isolated particles having bright and dark parts are finally formed [14, 17, 32, 33].
Similarly, the dark parts in Fig. 3c and the EDS results in Fig. 3f indicate that the Si diffusion happens at 800 °C in the oxidation atmosphere, and Au–Si droplets also form by Au/Si interdiffusion. In such an oxygen-rich environment, the Si passive oxidation easily occurs and the decomposition of the substrate is prevented. The diffused Si atoms are oxidized to SiOx on the outer surface of the droplets and the oxidation rate is much faster than the reducing counterpart, resulting in the formation of the thicker SiOx covering layers. In addition, a high temperature of 1050 °C can largely activate the Si diffusion, resulting in a heavy supersaturation of Si concentration in Au–Si droplets. As a result, extra Si precipitates and SiOx NWs nucleate and grow from Au–Si droplets based on the solid–liquid-solid mechanism [13]. Finally, Au/Si phase separation also happens upon cooling and the structures with bright and dark parts are formed.
Conclusions
Interesting structures obtained by annealing of Au thin films deposited onto Si substrates with a native oxide layer are carefully checked for different temperatures and ambient atmospheres. Low annealing temperatures of 500 °C and 650 °C only induce the initiation of voids and branched void growth in the Au thin films, respectively, and the annealing atmosphere shows negligible impact on the structure evolution due to limited Si diffusion. By contrast, the further increase of annealing temperatures enhances the material retraction and the Au/Si interdiffusion, accelerating the dewetting process and also leading to the formation of Au–Si droplets sinking into the substrate. The diffused Si in the Au–Si droplets at 800–1050 °C can be easily oxidized in Ar + O2 atmosphere according to the Si passive oxidation process, which results in the formation of a thick SiOx covering layer, and a temperature of 1050 °C can even activate the outward growth of free-standing SiOx NWs from the droplets. Similar Si passive oxidation can also happen during the annealing at 800 °C in Ar + H2 atmosphere, forming the stripe-like SiOx areas connecting many isolated particles. However, the occurrence of SiO2 decomposition and Si active oxidation at 950–1050 °C in Ar + H2 atmosphere prevents the formation of solid oxidation products. As a result, no SiOx structures form at holding temperatures, and only isolated particles presenting internal contrasts form due to the phase separation during the cooling period. Besides, the weak surface diffusion at 950 °C enables the Au to be mainly enriched within particles and the SiO2 decomposition is only locally enhanced around these particles. Together with further Si active oxidation within the decomposed areas, a clear cavity is formed around each particle. By comparison, the much stronger surface diffusion indicated by the presence of Ostwald ripening at 1050 °C results in the random distribution of decomposition areas, and no clear cavities can be observed.
References
Lenardi C, Mayer J, Faraone G et al (2020) Nanoscale-induced formation of silicide around gold nanoparticles encapsulated in a-Si. Langmuir 36:939–947. https://doi.org/10.1021/acs.langmuir.9b02993
Batič BŠ, Verbovšek T, Šetina J (2017) Decomposition of thin Au films on flat and structured Si substrate by annealing. Vacuum 138:134–138. https://doi.org/10.1016/j.vacuum.2016.12.006
Kang M, Yuwen Y, Hu W et al (2017) Self-organized freestanding one-dimensional Au nanoparticle arrays. ACS Nano 11:5844–5852. https://doi.org/10.1021/acsnano.7b01479
Oh H, Pyatenko A, Lee M (2019) Laser dewetting behaviors of Ag and Au thin films on glass and Si substrates: experiments and theoretical considerations. Appl Surf Sci 475:740–747. https://doi.org/10.1016/j.apsusc.2019.01.055
Hijazi H, Leroy F, Monier G et al (2020) Dynamics of gold droplet formation on SiO2/Si(111) surface. J Phys Chem C 124:11946–11951. https://doi.org/10.1021/acs.jpcc.0c02378
Panciera F, Tersoff J, Gamalski AD et al (2019) Surface crystallization of liquid Au–Si and its impact on catalysis. Adv Mater 31:1806544. https://doi.org/10.1002/adma.201806544
Okamoto H, Massalski T (1983) The Au− Si (gold-silicon) system. Bull Alloy Phase Diagr 4:190–198. https://doi.org/10.1007/BF02884878
Panciera F, Norton MM, Alam SB, Hofmann S, Molhave K, Ross FM (2016) Controlling nanowire growth through electric field-induced deformation of the catalyst droplet. Nat Commun 7:12271. https://doi.org/10.1038/ncomms12271
Kim S, Hill DJ, Pinion CW, Christesen JD, McBride JR, Cahoon JF (2017) Designing morphology in epitaxial silicon nanowires: the role of gold, surface chemistry, and phosphorus doping. ACS Nano 11:4453–4462. https://doi.org/10.1021/acsnano.7b00457
Curiotto S, Leroy F, Cheynis F, Muller P (2015) In-plane Si nanowire growth mechanism in absence of external Si flux. Nano Lett 15:4788–4792. https://doi.org/10.1021/acs.nanolett.5b01880
Li A, Zhao X, Anderson S, Zhang X (2018) Silica nanowire growth on coscinodiscus species diatom frustules via vapor-liquid-solid process. Small 14:1801822. https://doi.org/10.1002/smll.201801822
Shakthivel D, Navaraj WT, Champet S, Gregory DH, Dahiya RS (2019) Propagation of amorphous oxide nanowires via the VLS mechanism: growth kinetics. Nanoscale Adv 1:3568–3578. https://doi.org/10.1039/c9na00134d
Paulose M, Varghese OK, Grimes CA (2003) Synthesis of gold-silica composite nanowires through solid-liquid-solid phase growth. J Nanosci Nanotechnol 3:341–346. https://doi.org/10.1166/jnn.2003.209
Li F, Oliva-Ramírez M, Wang D, Schaaf P (2022) Effect of SiO2 interlayer thickness in Au/SiO2/Si multilayer systems on Si sources and the formation of Au-based nanostructures. Adv Mater Interfaces 9:2101493. https://doi.org/10.1002/admi.202101493
Tromp R, Rubloff GW, Balk P, LeGoues FK, van Loenen EJ (1985) High-temperature SiO2 decomposition at the SiO2/Si interface. Phys Rev Lett 55:2332–2335. https://doi.org/10.1103/PhysRevLett.55.2332
Leroy F, Passanante T, Cheynis F, Curiotto S, Bussmann EB, Müller P (2016) Catalytically enhanced thermal decomposition of chemically grown silicon oxide layers on Si(001). Appl Phys Lett 108:111601. https://doi.org/10.1063/1.4941799
Li F, Oliva-Ramírez M, Wang D, Schaaf P (2021) Formation and evolution of Au-SiOx heterostructures: from nanoflowers to nanosprouts. Mater Des 209:109956. https://doi.org/10.1016/j.matdes.2021.109956
Bhatta UM, Rath A, Dash JK et al (2009) Oxide mediated liquid-solid growth of high aspect ratio aligned gold silicide nanowires on Si(110) substrates. Nanotechnology 20:465601. https://doi.org/10.1088/0957-4484/20/46/465601
Kim TH, Shalav A, Elliman RG (2010) Active-oxidation of Si as the source of vapor-phase reactants in the growth of SiOx nanowires on Si. J Appl Phys 108:076102. https://doi.org/10.1063/1.3488882
Luo H, Wang R, Chen Y et al (2013) Enhanced photoluminescence from SiOx–Au nanostructures. CrystEngComm 15:10116–10122. https://doi.org/10.1039/c3ce41455h
Hofmann K, Rubloff G, Liehr M, Young D (1987) High temperature reaction and defect chemistry at the Si/SiO2 interface. Appl Surf Sci 30:25–31. https://doi.org/10.1016/0169-4332(87)90069-9
Bábor P, Duda R, Polčák J et al (2015) Real-time observation of self-limiting SiO2/Si decomposition catalysed by gold silicide droplets. RSC Adv 5:101726–101731. https://doi.org/10.1039/c5ra19472e
Herz A, Wang D, Kups T, Schaaf P (2014) Solid-state dewetting of Au/Ni bilayers: the effect of alloying on morphology evolution. J Appl phys 116:044307. https://doi.org/10.1063/1.4891448
Nsimama PD, Herz A, Wang D, Schaaf P (2016) Influence of the substrate on the morphological evolution of gold thin films during solid-state dewetting. Appl Surf Sci 388:475–482. https://doi.org/10.1016/j.apsusc.2015.11.185
Herz A, Theska F, Rossberg D, Kups T, Wang D, Schaaf P (2018) Solid-state dewetting of Au–Ni bi-layer films mediated through individual layer thickness and stacking sequence. Appl Surf Sci 444:505–510. https://doi.org/10.1016/j.apsusc.2018.03.096
Müller CM, Spolenak R (2010) Microstructure evolution during dewetting in thin Au films. Acta Mater 58:6035–6045. https://doi.org/10.1016/j.actamat.2010.07.021
Thompson CV (2012) Solid-state dewetting of thin films. Annu Rev Mater Res 42:399–434. https://doi.org/10.1146/annurev-matsci-070511-155048
Kosinova A, Kovalenko O, Klinger L, Rabkin E (2015) Mechanisms of solid-state dewetting of thin Au films in different annealing atmospheres. Acta Mater 83:91–101. https://doi.org/10.1016/j.actamat.2014.09.049
Shao YM, Nie TX, Jiang ZM, Zou J (2012) Behavior of Au-Si droplets in Si(001) at high temperatures. Appl Phys Lett 101:053104. https://doi.org/10.1063/1.4739413
Yao S, Wen M, Zhu GZ (2020) Bimodal size distribution of dewetted gold nanoparticles with regrown oxide bases. Appl Surf Sci 501:144227. https://doi.org/10.1016/j.apsusc.2019.144227
Dallaporta H, Liehr M, Lewis JE (1990) Silicon dioxide defects induced by metal impurities. Phys Rev B 41:5075–5083. https://doi.org/10.1103/PhysRevB.41.5075
Ressel B, Prince KC, Heun S, Homma Y (2003) Wetting of Si surfaces by Au-Si liquid alloys. J Appl Phys 93:3886–3892. https://doi.org/10.1063/1.1558996
Ferralis N, Maboudian R, Carraro C (2008) Temperature-induced self-pinning and nanolayering of AuSi eutectic droplets. J Am Chem Soc. https://doi.org/10.1021/ja7101983
Acknowledgements
The work was supported by the Deutsche Forschungsgemeinschaft (DFG, grant Scha 632/20, “FunPartY” and grant Scha 632/24, “Tailored Disorder” and Scha 632/27, “DFG-Gerätezentrum”). This work was also supported by the free state of Thuringia under grants 2015 FGI 0025 305 (FastµXRD) and B715-10009 (BioMacroNano2020), all co-financed by the European Union within the framework of the European Regional Development Fund (ERDF). Joachim Döll from the Center of Micro- and Nanotechnology (ZMN), a DFG-funded core facility at TU Ilmenau, is acknowledged for his professional help in the preparation of the samples. Sobin Mathew also helped with the spin coatings. For STEM and EDX-analyses we kindly acknowledge the Center for Electron Microscopy (ZELMI) of Technical University Berlin and the DFG for founding the JEM-ARM300F2 instrument (GZ: INST 131/789-1 FUGG). Financial support by the German Research Foundation (DFG) through Grant Reference Number STR 596/12-1 (as part of PAK XX), the federal ministry for education, research and development (Bundesministerium für Bildung und Forschung, BMBF) under Grant numbers 03SF0613D and 03SF0611A in the collaborative research projects “AEMready” and “H2-Meer” and the federal ministry for economic affairs and energy (Bundesministerium für Wirtschaft und Energie, BMWi) under Grant Number 03EIV041F in the collaborative research project “MethQuest” in the group “MethFuel” are gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Catalin Croitoru.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, F., Wang, D., Klingenhof, M. et al. Controllable Si oxidation mediated by annealing temperature and atmosphere. J Mater Sci 57, 10943–10952 (2022). https://doi.org/10.1007/s10853-022-07354-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07354-x