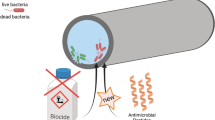
Abstract
Many corrosion problems are caused by the presence of microorganisms, so-called microbiologically influenced corrosion (MIC). A major representative of MIC-causing bacteria includes the group of sulfate-reducing bacteria (SRB), which accumulate in biofilms on the surface. Removal of biofilm inhabiting bacteria is much more complex compared to planktonic cell removal, in particular, based on the formation of an extracellular polymeric substance matrix (EPS layer). Current control strategies mainly involve the use of biocides. The development of resistance is a major problem caused by the limited number of suitable biocides and their frequent use. A consequence is a requirement of even elevated concentrations, which in worst-case scenarios results in a complete loss of efficacy. Recently, the use of antimicrobial peptides (AMPs) especially in the field of medical devices has been distinguished, including the coating of implants with AMPs for retarding or even completely preventing biofilm formation. Transferring AMPs to technical applications as MIC controlling agents offers high potential, therefore. However, based on open circuits, e.g. MIC on ship trunks or in wastewater pipes, immobilization of AMPs on surfaces is quite important, while keeping the AMPs active. This article presents various immobilization strategies established for this purpose, with a special focus on covalent AMP immobilization on metal surfaces.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ability of microorganisms to harm organic materials such as wood or plants has been known for a long time [1]. The fact that they also affect the integrity of metallic materials has been recognized quite lately; precisely since 2006, when a pipeline at the Prudhoe Bay oil field in central Alaska suffered from microbiologically influenced corrosion (MIC) [2]. MIC includes multiple bacterial species, where sulfate-reducing bacteria (SRB) are the best-studied [3]. In fact, they catalyze the initial reaction of MIC by converting sulfur at higher oxidation states (e.g., SO42−) to sulfur in lower oxidation states (e.g., S2−), generating the corrosion products FeS and Fe(OH)2 [4]. Alternatively, hydrogen sulfide (H2S) is formed. Sulfur at lower oxidation levels, such as S2- and hydrogen sulfide, gets oxidized to sulfuric acid by sulfur-oxidizing bacteria (SOB), where oxygen often serves as an electron acceptor. Sulfuric acid affects metals by acid corrosion and represents one of the reactants for SRBs [5, 6]. Consequently, symbiosis of SRBs and SOBs creates a cycle that is crucial for MIC damage.
Dou et al. report that 20% of total corrosion costs are caused by microbial attack [3]. Moreover, the corroded (construction) material causes environmental pollution leading to serious consequences for the whole ecosystem. Furthermore, the corroded (construction) material causes environmental pollution, leading to serious impacts on the whole ecosystem.
MIC phenomenon can arise quite anywhere: in oil and gas industry, in water and piping systems such as wastewater treatment or cooling circuits, on construction elements in marine environment, in nuclear power plants, in vehicles, as well as within the medical sector [3, 7]. In fact, MIC can affect any metal such as carbon steel [8], stainless steel [9], aluminum [10], copper [11], magnesium [12], zinc [13] and even titanium [14].
State-of-the-art in MIC management mostly includes the use of biocides. Based on the limited number of suitable biocides as well as their frequent use in particular within open systems, the development of resistance is a major problem. Consequently, increasing concentrations are required, accelerating the development of resistance [15, 16]. Recent research on MIC control is primarily focused on using compounds to potentiate biocidal effects, e.g. D-amino acids [8], proteins [17], chelates [18], or chelate–alcohol combinations [19]. Furthermore, inhibiting quorum sensing of participating bacteria is another approach to MIC control.
Based on nature, such an approach has been adopted since the red alga Delisea pulchra, in particular, lacks biofilm colonization despite the presence of numerous marine plants [20]. So the use of quorum sensing inhibitors as natural biocides against SRBs is highly obvious [21]. In addition, biofilms can be prevented by metal ions, commonly copper or silver, which are immobilized onto the material to be protected and released over time into the environment [22, 23]. This paper addresses the feasibility of using antimicrobial peptides (AMP) as highly efficient novel MIC control agents. AMPs are part of the natural immune response of nearly all organisms. Today, more than 3000 different peptide sequences with antimicrobial activity (showing main activity against bacteria) are known [24]. Among these, approximately 10% have already been proven to be active against biofilms [25]. Their mode of action can be classified into two basic principles: membranolysis and intracellular targeting. Membranolysis is based on electrostatic interaction of negatively charged phospholipids of bacterial membranes and positively charged AMP. This causes varying levels of attachment of the AMP onto the membrane and finally leads to their destruction (see also Fig. 4b). On intracellular targeting, the AMP targets a specific site within the cell (e.g., protein synthesis, nucleic acids) and modulates cell viability [26]. The use of AMPs to target bio-corrosive organisms has rarely been reported. Jayaraman et al. describe the application of AMPs produced by a host organism to avoid diffusive processes within a biofilm formed by SRB species (Desulfovibrio Vulgaris and Desulfovibrio Gigas). This approach, however, is limited by its host organism viability [27]. Despite the fact that the use of peptides for MIC treatment has rarely been discussed, the application of peptides in atmospheres where biocorrosion occurs has already been demonstrated. Peptides have been validated to be effective targeting a wide range of marine microorganisms including Vibrio natriegens, Shewanella putrefaciens, Halomonas aquamarina, as well as microalgae [28]. Latest research results show that cyclic peptides inhibit biofilm formation and eliminate existing biofilms using marine bacteria to protect against MIC and various marine biofilms in fouled industrial seawater reverse osmosis membrane. In a further experimental set-up, the efficacy of cyclic peptides was validated in solution as well as by covalent and non-covalent immobilization onto poly-methyl methacrylate [29]. Immobilization of peptides is quite significant toward successful implementation of AMPs to control MIC in open systems such as ship hulls and pipelines. Since various strategies for antimicrobial coating on medical devices such as implants have been well described [30, 31], transferring these immobilization methods toward an industrial application bears great potential.
This review addresses various AMP immobilization strategies. The main focus is placed on covalent AMP immobilization on metallic surfaces, as these materials are commonly targeted by biocorrosion. Specifically, the functionalization of metal surfaces and the various ways of contact killing using covalent immobilization on metal surfaces are described. Analytical methods for testing AMP immobilization are also reviewed. Furthermore, potential challenges and obstacles in establishing AMP coating as well as possible limitations and restrictions for immobilized AMPs in combating biocorrosion will be discussed.
Coating mechanisms
In general, coating mechanisms can be divided into passive and active modes of action. Passive coatings prevent the adhesion of bacteria while maintaining cell vitality. Adhesion can be prevented by various anti-adhesion strategies. For instance, highly hydrophobic surfaces that avoid adhesion by hydrophilic head groups of the lipid bilayer of the bacterial membrane, anionic surfaces that lead to electrostatic repulsion between surface and negatively charged bacterial membrane, as well as steric shielding [32]. Active coatings result in killing bacteria with antimicrobial substances. Such materials include the AMPs discussed here [33], as well as antibiotics e.g. vancomycin [34], metal ions e.g. gallium [35], and halogens e.g. iodine [36]. Active coatings can be divided into two groups: Active coatings can be divided into two groups: First, there are coatings with antimicrobial drug release. For this purpose, carrier media such as polymethyl methacrylate [33] are used, into which the active ingredients are mixed. Optionally, polyelectrolyte multilayers can be used, consisting of alternating layers of contrary charges, e.g. chitosan ( +) and hyaluronic acid (-), into which the active ingredients are added [37]. That method is based on the continuous release of antimicrobial substance(s) from the matrix. The drawback is that the substances are released into the environment at any time, even if this is not necessary. Moreover, the period of efficacy of that approach is limited. Whereas, there are coatings that are based on contact killing. Using this approach, antimicrobial substances get immobilized onto the surface and kill microorganisms via contact. These substances can either be coupled physically or chemically. Physical immobilization is based on non-covalent attachment. For this type of binding, the amino acid l-3,4-dihydroxyphenylalanine (l-DOPA) is often used, which is able to bind to surfaces via various mechanisms, e.g. hydrogen bonds, coordinative bonds with metal ions, or metal oxide surfaces, π-π-electron interactions as well as cation-π interactions [38]. Contrary to physical immobilization, chemical immobilization is based on forming covalent bonds. Here, binding takes place using functional groups that are already present, e.g. by linking the carboxylic peptide group to an amide group located on the surface, e.g. peptide BP100 (non-selective bond) [39]. Selective binding using thiol groups can be obtained by modifying a peptide, e.g., by insertion of cysteine. Such highly specific directional immobilization remains identical for all peptide molecules [40]. The drawback of selective binding consists in the fact a cysteine with a thiol group is not present in every AMP, and inserting an additional amino acid may significantly affect antimicrobial activity [41]. The key issue for contact killing is therefore that efficacy of an antimicrobial substance(s) must be retained despite immobilization. The use of linkers, e.g. polyethylene glycol (PEG), has proven beneficial for this purpose, as they create additional space between material surface and active ingredient while increasing flexibility of the drug [42]. Covalent linkage results in stable coatings, making them the most appropriate option for technical applications. Coatings used in technical applications, e.g. to protect ship trunks, have to resist several potent stresses (ocean waves, seabed, harbor walls). Consequently, the next chapter of this review will focus on contact kill strategies of AMPs on metal surfaces (Fig. 1).
Mechanisms of coating: passive action, whereby bacterial adhesion is prevented by an anti-adhesion effect caused by increased hydrophobicity, anionic surface, or steric shielding; active action, wherein bacteria are killed either by release or by direct contact with an antimicrobial substance (red bacteria)
Covalent immobilization
Using protective coatings based on AMP immobilization onto metals, no functional groups are existing at the surface compared to other materials like PEG. So the metal has to be functionalized first, followed by immobilization of active ingredients by various techniques.
Metal functionalization
Several ways of adding functional groups to a metal surface exist:
Plasma activation
Dielectric barrier discharge is one common method of plasma activation. This technique uses a chamber located between two electrodes which are separated by a dielectric barrier to create a plasma state when applying an alternating voltage across the electrodes. Using this method, stainless steel can be functionalized with Ar-O2 plasma at 30 W for 30 s in a continuous discharge mode [43]. Nickel nanoparticles can also be functionalized using helium gas via a glow plasma fluidized bed [44]. Duday et al. [45] described an approach where the plasma is produced using N2 (90 l/min), followed by a secondary step using N2 as carrier gas with (3-aminopropyl)trimethoxysilanes (APTMS) (50 l/min). Here, plasma activation is obtained by an after-annealing process using dielectric barrier discharge, where radiation is applied after removal of an ionization source. In comparison to normal dielectric barrier discharge processes, a smaller amount of amino groups is formed, however, the linkage shows increased stability. Furthermore, it has been proven that an amine-rich surface can be reached by plasma activation of cobalt-chromium (L605) using a mixture of N2/H2 in the first step at 150 W for 10 min at 100 mTorr followed by H2 at 150 W for 30 s at 300 mTorr within a second step [46]. Titanium could also be functionalized using O2 plasma for 10 min at 100 W [47]. Stainless steel (304 2B) was first cleaned by air plasma at 60 W for 1 min before being functionalized with allyl glycidyl ether monomer at 25 W for 1 min [48]. Benefits of this method include operating at atmospheric pressure, short treatment times lasting only a few minutes, e.g., 10 min for oxygen plasma treatment of titanium, [47], absence of strong chemicals, and large-scale process application [49]. Furthermore, different starting materials can induce different functionalization (e.g., oxygen plasma generates a hydroxy-rich surface where a N2/H2 plasma results in an amine-rich surface [46, 47]). Nevertheless, plasma activation requires special equipment, resulting in high investment costs. Also, the conditions of plasma activation must be adapted to each specific application (plasma, material, distance between electrodes and chamber, etc.), so initial tests must be carried out to define the effective dose [45, 50].
Plasma nitriding
In cases where a nitrogen-rich surface is necessary as starting material for immobilization, plasma nitriding can also be used. Here, the material is exposed to a nitrogen atmosphere at high temperatures allowing the nitrogen to diffuse into the material. This leads to a reaction of nitrogen and iron, resulting in formation of iron nitride (including Fe3N) [49].
A benefit associated with this method is that plasma nitride steel is already commercially available since this method is often used for hardening steel and no functionalization is required anymore in the laboratory. The drawback of this method is that high temperatures (700 °C) are required and immobilization can only be achieved under defined catalytic conditions [49] (see “Direct coupling” section).
UV/ozone
In this functionalization method, tantalum is placed in a UVO cleaner for 2 h [51] similarly titanium has been treated by a UV-Ozone Pro Cleaner device for 15 min [52]. As a benefit, this method has been proven to provide excellent functionalization for subsequent coupling of other molecules. Removing hydrophobic impurities, incorporating hydroxyl groups, as well as obtaining highly hydrophilic surface has been observed to be more efficient than chemical methods (HNO3) for coupling (3-aminopropyl)triethoxysilanes (APTES) [51]. The drawback is using ozone, a known powerful and toxic oxidant. Also, the acquisition of a UVO device must be considered. Compared to plasma activation, whereby material can be loaded with different functional groups, using UV/ozone technique only hydroxyl groups can be introduced.
Chemical functionalization
Besides the physical activation methods including plasma and UV/ozone, chemical approaches could also be considered. To insert hydroxyl groups, a so-called piranha solution containing concentrated sulfuric acid and 30% hydrogen peroxide was proven to be successful. Depending on the material used, different ratios are applied for this solution (7:3 for AISI 304 [53], 3:1 for SS316 [54], 1:1 for titanium [42]). The material is immersed in the solution at room temperature for one hour. Optionally, hydrogen peroxide can also be used to incorporate hydroxyl groups. This is done by immersing alumina in the solution at 100 °C for 15 min [39, 40, 55]. As a further chemical functionalization, 32.3% nitric acid (immersion time 10 min) can be applied to tantalum [51]. Alkaline solutions such as NaOH could also be used for introducing hydroxyl groups. Notable examples include immersion of magnesium AZ 31 at 80 °C for 12 h [55] as well as titanium at 80 °C for 24 h [56].
A benefit of chemical functionalization is its simplicity, as the metal is only immersed in a solution for a defined period of time [53]. Moreover, expensive equipment is not required when compared to plasma activation. Besides, as mentioned above, chemical functionalization can be performed on a wide range of materials (stainless steel [53], titanium [42], aluminum oxide [40]). One drawback of chemical activation is the need for often highly concentrated and aggressive chemicals like Piranha solution [53], which are not completely safe to handle and whose disposal can be environmentally critical.
Immobilization strategy
After successful functionalization of metals, AMP immobilization is performed using different strategies. Two types are described herein particular: First, covalent immobilization, in which the peptides are coupled via strictly covalent bonds (blue background in Fig. 2), and the second one, semi-covalent immobilization, where the peptides are covalently bound to a linker, whereas the linker is not covalently bound to the metal (green background in Fig. 2). Selection of the most suitable immobilization strategy requires attention to the most compatible peptide sequence (which functional groups are included in the peptide sequence to be used for coupling without loss of activity).
Overview of covalent immobilization strategies (blue): direct coupling via plasma nitriding, silanization via (3-aminopropyl)triethoxysilanes (APTES), 11-(2-bromo-2methyl)propionyloxyundecenyltrichlorosilane (BPTCS), silane with sodium azide, Polyethylene glycol (PEG), maleimide or cysteine, polymer brushes with poly-glycidyl methacrylate (PGMA); semi-covalent immobilization strategies (green): self-assembly monolayer (SAM) via 11-Mercaptoundecanoic acid (MUA) or peptide, nanotubes like carbon nanotubes, poly-l-DOPA (* selection of possible binding mechanisms, for details see “Polymerization of l-DOPA” section); respective functional groups for peptide coupling are colored
Direct coupling
Direct coupling chemistry requires activation chemicals (2-(1 h-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and N,N-diisopropylethylamine (DIEA). Therefore, HBTU in 1 mL of dimethylformamide (DMF) (0.052 M), 0.1 mL of DIEA, and the antimicrobial peptide (0.38 M) per square centimeter of surface area are allowed to react for 16 h under continuous stirring. At these conditions, nitrogen of iron nitride should also be able to interact with the peptide, resulting in a peptide bond formation between iron nitride and the peptide's carboxyl group. Peptide attachment leads to a 3-log reduction of bacterial counts within less than 45 min against various biofilm formers (E. Coli, P. aeruginosa, S. aureus, S. epidermidis, Enterococcus) [49].
The advantage of direct coupling method is the rapid procedure, whereby the material is simply immersed in peptide solution with continuous stirring for 16 h avoiding usage of a linker [49]. However, the linker represents a crucial factor for the flexibility related to the bound AMP. Based on the close linkage of AMP and the surface, their activity is restricted [48]. Moreover, direct coupling via nitriding steel requires special conditions created by powerful catalytic chemicals [49].
Silanization
To circumvent problems of direct coupling, silanization is often used. For this purpose, the material is immersed in a silane solution (e.g. APTES to generate an amine-rich surface). The process of attaching silanes onto functionalized surfaces is shown in Fig. 3. First, hydrolysis (1) takes place, cleaving off an ethyl residue. Thereafter, silane attaches to the hydroxylated surface (2) via hydrogen bonds. Finally, condensation (3) leads to a covalent bond, which links silane and surface.
Reaction of silane, here ((3-aminopropyl)triethoxysilanes (APTES), to hydroxylated surfaces by hydrolysis (1), adsorption (2), and condensation (3); adapted with permission from [57]. Copyright 2019, Carbohydrate Polymers
As mentioned above, APTES can be used to obtain an amine-rich surface. For this, aluminum nanoparticles are placed in a solution containing 7% APTES in toluene for 120 min. Afterward, the nanoparticles are added to a peptide solution containing a peptide (BP100) modified using a tag (EAAA). The solution is kept at 45 °C for 3 h allowing to obtain a peptide bond between the carboxyl group of the peptide and the APTES amine group [39]. Since direct coupling of peptides to a functional surface can also induce non-covalent interactions, like electrostatic attraction, however, this coupling method seems to be poorly stable [43]. To prevent this, activation of silane-generated functional groups is often implemented. For instance, SS AISI 304 stainless steel is coated with APTES (ATPES/toluene solution for 18 h under gentle shaking) activated via N-hydroxysuccinimide (NHS) by the catalytic activity of 4-(dimethylamino)pyridine. Subsequently, the samples are added to a peptide solution (50 µg/mL Magainin II) for 24 h with gentle shaking, resulting in a Schiff base reaction of Magainin II and the functionalized silanes [53]. Stainless steel (316L) is dipped in APTES, 2% v/v in toluene, allowed to react at room temperature for 1 h, and subsequently activated with 2 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and 5 mM NHS (15 min). Within this commonly used activation procedure, the carbodiimide carbon of EDC first couples to the carboxylic group, forming an unstable, reactive intermediate to which NHS finally binds. This results in a semi-stable NHS ester with one reactive amino group. Finally, this leads to formation of an amide bond between NHS ester and the peptide's amino group, cleaving the alcohol (amminolysis) [43]. Pre-activated stainless steel surface is submerged into a 1 mg/mL peptide solution (KLLLRLRKLLRR). Almost complete bacterial colonization (95–100%) with E. coli and S. aureus can be avoided [58]. APTES can also be applied to zinc oxide, whereby no material preparation is necessary, as an oxide layer is already provided. In this case, activation is also carried out with EDC/NHS [59]. Either, with titanium (Ti-6Al-4 V) a coupling with APTES and EDC/NHS activation takes place. The peptide KR-12 results in a high antibacterial activity of approx. 90% against S. epidermidis. A linker (PEG) could also be connected to bound APTES, as well as their amine terminus was also been activated by EDC/NHS. Based on the increased flexibility, an antibacterial effect against S. epidermidis of almost 100% can be achieved by this route. [56]. In addition to EDC/NHS activation, coupled APTES can also be used for peptide immobilization by coupling other chemicals. For this, the material (alumina) was incubated for 1 h at room temperature in DMF and N-succinimidyl-3-maleimidopropionate. Subsequently, immobilization is done by Michael addition of the peptides cysteine and maleimide [55]. Magnesium (AZ31) also undergoes peptide immobilization using the same procedure [60]. Beyond that, coupling of 3-(maleimido)propionic acid N-hydroxysuccinimide ester to APTES is also used. Peptides are coupled to tantalum [51] as well as to titanium [47] using this coupling strategy. Accordingly, each peptide is n-terminally modified using 3-mercaptopropionic acid and a 6-aminohexanoic acid tetramer, resulting in a nucleophilic substitution involving the immobilized iodine and the thiol group of the peptides, which leads to the binding of the peptides to the surface.
Besides APTES, APTMS can also create an amine-rich surface. Again, the surface is activated by EDC/NHS (0.2 M EDC and 0.1 M NHS) enabling nisin to be permanently coupled to 304 2B stainless steel [45].
Still, other silanes with functional groups exist, however. (3-Glycidyloxypropyl)-trimethoxysilane (GPTS), combined with PEG, is used for coating titanium bearing a carboxyl-rich surface, for example. To obtain AMP binding, initially, the carboxyl group must be activated by 0.1 M dicyclohexylcarbodiimide and 0.1 M NHS for 2 h in order to bind LL-37 by peptide linkage [42]. A carboxyl-rich surface can also be created using vinyltrimethoxysilane and maleic anhydrides. The surfaces must also be activated by EDC/NHS (4 mM EDC + 10 mM NHS). Subsequently, the AMP (5 mg/mL) can be linked. This results in a surface that causes eightfold logarithmic reduction of bacterial growth (B. subtilis) [43].
Besides carboxyl- and amine-rich silanes, epoxy-group-containing silanes, such as GPTS, can also be used. Following successful silanization of titanium, nucleophilic addition of the peptide's amino group (LL-37) to the epoxy group is finally carried out (see “Direct coupling” section) [42].
Silanization can also be used to generate thiol-rich surfaces. APTES, to which cysteine is coupled, can be used for that. In the case of a cysteine-containing peptide sequence or a cysteine-containing anchor sequence (as here: CAAA), the peptide can be coupled via disulfide bridge formation [40]. Alternatively, silanes with thiol groups such as 3-Mercaptopropyltrimethoxysilane can be used [61].
Furthermore, silanes such as 3-chloropropyltriethoxysilanes could also be paired with sodium azide. Modification of peptides (BP100 and DDK) with 2-aminopent-4-ynoic acid results in a copper(I)-catalyzed azide-alkyne cycloaddition [40].
Likewise, halogen-containing silanes, such as bromine-containing 11-(2-bromo-2-methyl)-propionyloxyundecenyltrichlorosilane (BPTCS), can be immobilized. Halogens serve as initiators of polymer brushes and allow their formation upon the immobilized silanes (see “Polymerization of l-DOPA” section for further details) [47].
Silanization offers the benefits of providing a wide range of different silanes with a variety of functional groups (carboxyl, amines, thiol, epoxy, azide, halogens) depending on the coupling mechanism selected [62]. Beyond that, silanization can be applied to many surface materials. While this method is applicable to metals, it can also be used to treat other materials like glass [63]. Compared to direct peptide coupling, silanization provides AMPs enhanced flexibility resulting in increased antimicrobial activity. Based on their length, PEG-coupled silanes offer additional flexibility that helps to improve antimicrobial activity [47]. Although silanization works for a wide variety of materials, there are still significant differences in silanization efficiency. While glass has the best efficiency, it decreases for aluminum, steel/iron, nickel, and zinc [63]. Moreover, functional groups need to be activated (for example via EDC/NHS activation) [58] or pre-functionalized by coupling other chemical compounds to form stable linkages [47], which is time and cost consumption. Insertion of a linker such as PEG helps to improve AMP efficacy while, in contrast, the step is time-consuming, costly, and prolongs AMP immobilization [56]. Consequently, silanization is a multistep immobilization process that requires additional chemical reagents for activation. Moreover, a hydroxylated surface is required for the coupling of silanes [57], meaning that functionalization of metals necessarily requires a hydroxylated surface.
Polymer brushes
Polymer brushes are tightly arranged copolymers that are separated by space constrictions between individual chains and its substrate. For example, nickel nanoparticles can be coated with acrylic acid by plasma polymerization. This results in a carboxyl-rich surface that needs to be activated with EDC/NHS to allow peptide immobilization. The activated nickel particles are finally immersed in 0.2 mg/mL peptide solution (LL-37) [44]. Titanium can first be coated with polyglycidyl methacrylate (PGMA) followed by polyacrylic acid. Activation with EDC and NHS is also necessary. As the coating is done with previously polymerized materials, this is referred to as " grafting to" method [64]. Atlas et al. did experiments wherein a bromine-containing compound (see “Polymer brushes” section) was coupled to l-DOPA to serve as an initiator for radical atom transfer polymerization of oligo-(ethylene glycol)methacrylate ("grafting to" method). Finally, succinic acid was coupled to these polymer brushes, whose carboxyl groups were pre-activated by EDC/NHS to produce the peptide bond [54]. While Atlas et al. used coupling with l-DOPA, Godoy-Gallardo et al. first silanised a titanium surface. Two strategies were used here: (1) APTES coupling followed by binding of 2-chloropropionyl chloride (2) BPTCS, both containing halogens as initiators for the subsequent polymerization of N,N-dimethylacrylamide and N-(3-aminopropyl)-methacrylamide ("grafting to"). Following this, the polymer brushes are crosslinked and functionalized with 3-(maleimido)-propionic acid-N-hydroxysuccinimide ester. Enabling nucleophilic substitution of the iodine and a thiol group, 3-mercaptopropionic acid is substituted at the N-terminus of the used hLf1-11 peptide [47]. Also, additional polymer brushes with polyglycidyl methacrylate (PGMA) and activation of the carboxyl group with EDC/NHS on titanium were done [52].
A major benefit of using polymer brushes is their multiple binding sites. Whereas silanes only allow one peptide attachment site, polymer brushes can attach a much higher quantity of peptides. This is reflected in antibacterial efficacy. While inhibition of bacterial adhesion of S. sanguinis and L. salivarius by hLf1-11 coupled via silanes ranges between 38–43%, it is significantly higher (58–72%) for hLf1-11 immobilized via polymer brushes [47]. A disadvantage of polymer brushes is the large number of different sub-processes. Initially, an activator to initiate the subsequent polymerization must be attached [47], alternatively, the polymer must be pre-polymerized in a complex process (plasma polymerization) [44]. This is often followed by activation of functional groups.
Polymerization of l-DOPA
Coating with polymerized l-DOPA can be achieved by various interactions. For one, non-covalent immobilization is possible via hydrogen bonds, π-π-electron interactions, cation-π interactions, complex formation with metal ions, hydrophobic or electrostatic interactions [38, 65]. There is no need for any special surface functionalization and it can be applied to a wide range of different materials [66]. However, a covalent coating with poly-l-DOPA can also be generated. Requirements are functional groups, that have to be provided. Immobilization of l-DOPA catechol is achieved by formation of amides on amine-rich surfaces, formation of urethanes on carboxyl-rich surfaces, or formation of ester bonds on hydroxyl-rich surfaces [38]. Otherwise, linking quinone compound via Schiff base or Michael addition to an amino-rich surface for covalent immobilization is also possible [67]. The coupling strategy of l-DOPA and peptide can be divided into two strategies. First strategy is to immobilize poly-l-DOPA on the surface, followed by coupling the peptides to poly-l-DOPA via Schiff base reaction using quinone and Michael addition involving catechins [68]. This method is carried out with magainin II [69], various cyclic peptides [70], or 2 synthetic peptides (P1 = ACTSNADNKYLPKTCQT P2 = ACTFFAFFFYLPFTCFT) [71] on stainless steel. Here, 40 µg/mL is given as the optimal l-DOPA concentration, since at higher concentrations self-polymerization of individual l-DOPA molecules dominates, leading to a reduction in surface binding [71]. Nevertheless, the polymerization of l-DOPA can also act on different materials such as cobalt-chromium. For this, surface passivation with 40% HNO3 for 40 min is first done. Subsequently, the material is placed in 10 mM TRIS + 2 mg/mL l-DOPA, pH 8.5, overnight in order to provide the weakly alkaline conditions required for polymerization. Subsequently, the material is immersed overnight at room temperature [71] or for 24 h [72] in a 1 mg/mL peptide solution. Second strategy involves firstly coupling of peptides to l-DOPA, followed by polymerization of l-DOPA and its binding to the material. For this purpose, 0.8 mg/mL Magainin II is first incubated with 8 mg/mL l-DOPA at room temperature using the activation reagent EDC (8 mg/mL) with gentle shaking. The coated steel surfaces show high antibacterial efficacy in reducing bacterial growth in the follow-up antimicrobial test involving seawater bacteria: Vibrio natriegens: 99.79%, Citrobacter farmer: 99.33% [72]. This second coupling strategy (1. Peptide coupling, 2. polymerization) results in higher peptide densities so the slightly increased AMP activity compared to the first coupling strategy, (Vibrio natriegens: 98.07%) can be explained [69]. Thus, a very efficient AMP immobilization strategy could be implemented to prevent marine biofilm formation.
Instead of direct immobilization of peptides to poly-l-DOPA, other chemicals can also be coupled to the poly-l-DOPA layer. This is done by Alas et al. who immerse the poly-l-DOPA-coated stainless steel samples in a 19 molar 2-bromoisobutyryl bromide solution [54]. This halogen compound serves as an initiator for polymer brushes (see “Polymerization of l-DOPA” section).
Advantage of this immobilization method is it is relatively simple and can be performed in only a few steps. Additionally, no activation chemicals are needed, especially for the first strategy [69,70,71]. Furthermore, this method can be used with a variety of different materials. [73] For covalent immobilization onto material surfaces, l-DOPA can interact with different functional groups on the surface like amine, carboxy, or hydroxy groups [38]. This makes surface functionalization much more flexible compared to other methods, such as silanization, which requires hydroxy groups [57]. A further advantage is a powerful attachment, which remains even after some time [69, 72]. The drawback of this method is that polymerization takes place only under special conditions (oxygen-rich, slightly alkaline environment), meaning parameters must be set for these conditions to ensure successful coating. [71] Although the strategy of first coupling the peptide to l-DOPA followed by polymerization seems more advantageous initially, since higher activity is obtained based on the higher peptide density, this procedure is more complex and expensive [69].
Self-assembly monolayer
A layer with functional groups can also be generated via the self-assembly monolayer (SAM) method. Here, spontaneous formation of a layer occurs when a surface is immersed in a solution containing self-assembling molecules. Peptide assembly is a spontaneous thermodynamic and kinetic driven process, based on the synergistic effect of various intermolecular non-covalent interactions, including hydrogen bonding,
π–π stacking, electrostatic, hydrophobic, and van der Waals interactions. These interactions maintain the peptide-based self-assembled structures in a stable low-energy state [74].
The attachment of peptides to a surface can be realized by those different non-covalent interactions. One way to attach peptides onto metal surfaces can be achieved by chemisorption, which results in binding between a thiol-containing substances and the surface, with SAMs on gold being the best characterized [75, 76]. An example of generating antimicrobial coatings is provided by studies using the peptide maginin I. Here, a gold coating is first placed in a solution of 11-Mercaptoundecanoic acid (MUA) for 3 h. The resulting carboxyl-rich surface is activated using the EDC/NHS method (10 mM EDC + 20 mM NHS). This is followed by coupling Magainin I at room temperature for 2 h [77]. Instead of coupling a peptide to an existing self-assembly, peptides, such as Pac-525, also exist that can form SAM by themselves. Therefore, the gold is immersed in a solution containing 0.6 mmol/l peptide and incubated at 37 °C [76].
The advantage of using the SAM technique is that no major pre-treatment is necessary. The gold surface is just annealed with a butane flame for good crystallinity [77] or cleaned in 75% ethanol solution for 30 min [76]. Moreover, the SAM method is a simple and energy-efficient method, especially when the peptides directly perform self-assembly. That means that the coating can be done as a one-step process [76]. Even after 6 months, there is still good provable antibacterial activity, however with a slight decrease [77]. However, stability of self-assembly of molecules depends on its own characteristics, as mentioned above. Similarly, environmental conditions such as pH value, temperature, and ionic strength affect the stability of nanostructures. Above all, an important role is attributed to the thiol compounds [76, 77]. Using the SAM method, however, the material takes a very central role. Many studies focus on SAM upon gold [54], however, steel and aluminum play a much more decisive role in technical applications [78].
Nanotubes
The stainless steel is incubated with acetylene at 700 °C for 5 min, which serves as a carbon source for nanotubes [79]. Here, several acetylene molecules cause a ring closure using metal catalysts [80], whereby in this case the metal catalysts used are iron derived from the material to be coated. Nanotubes are treated with 3 M nitric acid and modified by oxidation to form carboxyl groups at the ends as well as at missing sites on the sidewalls. Subsequently, they are activated with 1-ethyl-3(3-dimethylamino-propyl)-carbodiimide to form present carboxyl groups. The coupling of the peptides is achieved via ammonolysis, caused by the amine residues of the lysine [79].
One benefit of nanotubes is their highly mechanically stability. Relative to their weight, they are much more resilient (at least 333 times) than stainless steel [81]. One drawback of producing carbon nanotubes is that very high temperature (700 °C in this example) are required [79]. Moreover, nanotubes have to be modified to carry out functional groups, which also have to be activated [79]. Accordingly, nanotube formation represents a multistep process requiring several activation pathways.
Further linker options
After considering multiple coupling options, this section discusses additional linkers. After producing an amine-rich surface by plasma activation (N2/H2), glutaric anhydride is used as a linker. For this, the surface is immersed in 0.1 g/mL of glutaric anhydride solution for 1 h. Next, a carboxylic anhydride is formed by this linker. After that, a carboxyl-rich surface is achieved using this linker, requiring additional activation (3 × for 10 min in 3 mg/mL EDC in 2-(N-morpholino)-ethanesulfonic acid, pH 4.75) for peptide binding. Finally, the activated surface is placed in peptide solution for 3 h for peptide bond formation [46]. Another coupling strategy uses continuous plasma polymerization for coating stainless steel (SS 304 2B) coupons with allyl glycidyl ether monomer. Immobilization is achieved by nucleophilic addition of N-terminal amino groups or amino side groups (lysine or arginine) to the resulting epoxide group. This leads to cleavage of the epoxide group and binding of the peptide simultaneously. Peptides coupled in this way (nisin, palmitoyl-4 K, tritrpticin) decrease bacterial growth of E. coli and B. subtilis significantly by 3–6 log-steps compared to uncoated surfaces [48]. Similar to other linkers, the linker used here also increases the flexibility of the peptide [47]. However, an additional activation of the linker is necessary, which causes further steps [45].
A complete overview of previous immobilization can be found in Table 1.
Immobilization analysis
A large number of methods are available and must be selected according to parameters of interest. In this section, a selection of different analytical techniques is discussed.
For instance, AMP immobilization can be verified by surface characterization such as water contact angle via physicochemical parameters of the peptide [58]. An advantage associated with the contact angle measurement is its simplicity, for which only a small amount of sample is required. This measurement is based on the determination of hydrophobicity, meaning that immobilization of a peptide can be assessed based on changes in hydrophobicity. It is not possible to differentiate whether the change in hydrophobicity is caused by covalent or non-covalent immobilization of the peptides. Indeed, it is not possible to perform a complete peptide analysis because the peptides involved have similar hydrophobicities, indicating that contact angle analysis might not completely characterize the surfaces [84]. The layer thickness can be determined by surface plasmon resonance spectroscopy (SPR) with an imaging addition [85] or ellipsometry [47]. Whereas the last-mentioned technique is more accurate and sensitive in direct comparison [86], which results from signal interferences caused by nonspecific binding in SPR [85]. Quartz crystal microbalances with dissipation monitoring (QCM-D) can also be used to determine adsorption and binding kinetics. In contrast to optical methods such as SPR or ellipsometry, QCM-D determines the mass of the adsorbed film including the solvent [29]. Fourier-transformed infrared spectroscopy (FT-IR) identifies a substance by comparison with a reference spectrum [43]. However, within FT-IR measurements, further investigations are necessary for a quantitative statement [87]. This can be done via X-ray photoelectron spectroscopy (XPS) [48] or energy-dispersive x-ray spectroscopy (EDX) [79] using element analysis. The huge advantage of these two methods is the quantitative identification. However, this process is considered to be costly and time-consuming as well as requiring high-vacuum [88]. X-ray diffraction (XRD) results in a structural analysis [40]. This method also leads to a quantitative statement, but initially, the sample has to be transformed into its crystalline structure. Moreover, intensity of XRD is significantly lower compared to electron diffraction [89]. Time-of-flight secondary ion mass spectroscopy (ToF–SIMS) is a quantified identification based on mass [49] but is limited by long analysis time and non-simultaneous analysis of non-metal and metal elements [90]. Additional, different images of coated surfaces can be taken using different microscopy techniques: scanning electron microscopy (SEM) [43], atomic force microscopy (AFM) [43], and transmission electron microscopy (TEM) [40]. Major advantage of AFM and TEM is its high resolution, which leads to extensive data coverage with respect to chemical and physical parameters while requiring only small quantities of sample. However, these advantages can also cause negative effects, since the evaluation of just a small section does not necessarily have to be representative of the whole sample [91]. Avoiding this requires taking multiple exposures at different points on the object, resulting in additional effort.
The complete list of analysis used within different immobilization techniques is summarized in Table 1.
Furthermore, it should be mentioned that the majority of these analytical techniques have been established and applied only on laboratory scale so far. For large-scale application as well as industrial scale, adaptation strategies have to be considered.
Stability
Stability of peptide immobilization becomes crucial in order to provide long-term protection of the material. Nevertheless, stability studies have already been performed for some of the described immobilization techniques (see Table 1).
In case of direct linkage to epoxy layers, constant peptide activity can be obtained for 24 h even after immersion the coated stainless steel in water as well as non-ionic detergents at neutral pH [48]. Similar results were obtained for plasma-silanised stainless steel samples. Cleaning with a sponge dipped in the relevant solution also causes no loss of activity [45]. Immersion in PBS buffer for 1 h also causes no change in antimicrobial activity [43]. However, the antibacterial efficacy of silanized peptides, as well as peptides coupled by polymer brushes, decreased by up to 70% after immersion in PBS followed by 2 h of ultrasonication [47]. Plasma nitration also appears to be stable, as no change in antimicrobial activity was observed after exposure to zirconium oxide beads. In this case, the beads are exposed to peptide-functionalized material twice per second over a period of 72 h [49]. Stainless steel surfaces polymerized with l-DOPA are also tested for stability. Moreover, this was already tested in a technical application using seawater, showing a slight decrease in activity. Coupling the peptide first with l-DOPA followed by immobilization improved the antibacterial activity (from 99.8% to 93.0% after 28 days) compared to first polymerization followed by peptide coupling (from 98.1% to 85.2% after 28 days). Thereby higher peptide densities and a more homogeneous surface are obtained [69]. Further tests confirmed that a minor loss of activity occurs only within the first few days after immersing the material in water or seawater while gently shaking it. Thereby, poorly/lightly bound peptides are removed, whereas a constant peptide efficacy can be observed within the following days [53, 72]. Long-term studies have been scarce, but tests performed up to now seem promising, especially since immobilized Magainin I remains effective even after 6 months [77]. Accordingly, based on the present stability studies, its successful application in technical applications is to be expected. Notably, the long-term effect of other processes, such as the immobilized metal ion layer, is limited by the release of the ions. Admittedly, a linear increase in copper release was observed up to 20th days, but afterward saturation of the released copper ions can already be observed [22]. Even the release of silver ions immobilized on iron oxide indicates that half of all possible metal ions have already passed the depot after 20 days [23]. Additionally, silver, which can be oxidized quickly, shows low stability and long-term activity [92], meaning that AMP immobilizations certainly have advantages regarding long-term protection.
Challenges and critical aspects
Although successful AMP immobilization on metal surfaces could be documented within the abovementioned examples, several obstacles exist to transferring it to technical applications. In contrast to polymers, where AMPs can be directly linked based on pre-existing functional groups [93], covalent immobilization on metallic surfaces initially requires a functionalization step to introduce functional groups (see “Metal functionalization” section). Direct coupling step was tested with nitrided steel. However, peptide immobilization could only be carried out in strictly defined conditions using strong catalysts [49]. Another issue concerns that immobilized AMPs need to be flexible for interacting with bacterial membranes (see Fig. 4). Flexible linkers such as silanes or polymeric binders are often used for this purpose, providing space between surfaces and functional AMPs. This allows improved binding of cationic amino acids to negatively charged bacterial membranes [48]. In addition, AMPs require space between each other to be able to form its secondary structures. Consequently, a small AMP concentration may have advantages regarding improved antibacterial efficacy compared to a surface coated extensively with AMPs [93].
Apart from linkers and AMP density, orientation of AMPs is also essential. In case of non-specific immobilization, peptides may have different orientations at the surface (see bright peptide in Fig. 4a). Depending on the orientation, activity may be reduced or even lost completely [29]. However, specific binding is not always possible because amino acids with specific functional groups are missing and insertion of these amino acids can lead to a reduction in activity, so the insertion of cysteine reduces the effect by half [41]. The next challenge involves the costs. In technical applications, larger surface area has to be coated compared to medical applications [27], therefore the cost has to be minimized making the coating profitable for industry. Keeping the cost of peptide synthesis at a minimum, basic amino acids and low numbers of diverse amino acids can be used like it is done for "LK" peptides, composed of lysine and leucine with occasional incorporation of tryptophan [94, 95]. The use of natural amino acids enables AMPs to be degraded by proteolysis. In order to overcome this problem, a number of structural changes and modifications can help. These include replacing partly or completely the naturally occurring l-amino acids with their D-form. Pandinin-2 has been reported to lose its antibacterial activity when exposed to proteases, while the corresponding D-sequence retains its antibacterial activity [96]. Similarly, an increased protease stability of the peptide Pep05 could be obtained by replacing l-lysine and l-arginine with their respective D-forms. Likewise, Pep05 was also able to achieve increased protease stability by incorporating unnatural amino acids such as l-2,4-diaminobutanoic acid, l-2,3-diaminopropionic acid, l-homoarginine, 4-aminobutanoic acid, and l-thienylalanine [97]. Besides, the incorporation of the three arginine derivatives namely l-2-amino-3-guanidinopropionic acid, l-2-amino-4-guanidinobutyric acid, and l-homoarginine as well as three lysine derivatives namely l-2,3-diaminopropionic acid, l-2,4-diaminobutyric acid and l-ornithine can increase protease stability of peptide 1018, wherein lysine derivatives provide stability enhancement in particular [98]. Modifying the N- and/or C-terminus by N-acetylation and C-amidation represents another frequently documented technique [99]. Additionally, further peptide modifications can be done, such as replacing carbonyl groups with sulfur, phosphorus, or boron compounds, substituting nitrogen with sulfur or oxygen, or even cyclization [100]. Despite all countermeasures by an appropriate peptide design, it should be clear that there can be no unlimited effective lifetime. However, to make AMP immobilization still profitable, it should be ensured that the peptides remain stable for as long as possible. While stability studies have been performed in some cases, they often can be observed for a limited period of time. (maximum 28 d [69]). However, the maximum effectiveness of the immobilized peptides should be determined in any case. Even if this is limited, a retarding of biocorrosion is already an approach. Coated pipelines, for example, must be replaced significantly seldom compared to a pipeline without AMP coating, resulting in a cost reduction. This has already been successfully demonstrated with peptide A from sea anemone, which significantly retarded biocorrosion over 14 days, resulting in lower biocide application [101]. Realizing immobilization of AMPs for industrial applications is a challenge, considering that there are different technical fields that demand different requirements and surface materials. To address this, different immobilization strategies exist, so the most appropriate one must be selected depending on the application. Major factors for selection are the functional groups of the peptide. Therefore, choosing the best immobilization strategy requires considerations not only with respect to the material itself but also regarding the peptide to be immobilized. Modifications of the peptide structure for selective coupling should only be combined with a stringent screening of its antimicrobial activity.
Membrane targeting of AMPs impossible interaction between the immobilized AMPs and negatively charged bacterial membranes caused by very tight immobilization of AMPs and lack of linkers (a) or no antimicrobial activity based on incorrect conformation of the peptide (light orange peptide in a), flexible AMPs caused by linkers (yellow), allowing attachment to bacterial membranes, leading to membrane destruction (b)
As discussed in this section, there are many problems to be solved in establishing an AMP-immobilized method for protecting metallic surfaces in industrial applications.
Conclusion
Although there are plenty of obstacles to overcome in order to successfully establish an AMP coating in technical applications, immobilizing AMP on metal surfaces is possible, as shown by the various examples presented in this review. However, immobilization does not occur without functionalization of the metal. Antibacterial activity of the immobilized peptides against various biofilm-forming bacteria is reported in nearly every study, resulting in significantly reduced biofilm formation. In addition, the stability studies already performed are promising. Thus, broadening AMP immobilizations for protection metal surfaces versus biofilms in technical applications is feasible. Unlike the medical field, other species of bacteria, such as sulfate-reducing bacteria (SRB) like Desulfovibrio spp. and sulfur-oxidizing bacteria (SOB) like Thiomonas spp., are also involved in biofilm formation within industrial applications. Both of these bacterial species, among many others, are mainly responsible for microbiologically-influenced corrosion (MIC), which causes tremendous damage to various plants and facilities [3]. In consequence, using immobilized AMPs provides an environmentally friendly alternative to current MIC prevention. In addition to eco-compatibility of the AMP-based method, there is another major advantage of peptide coating: based on plenty of known AMPs or by generating new AMP sequences, almost infinite options are available to provide suitable AMP sequences related to the bacteria to be killed. Modifications of the peptide caused by instabilities or resistance developments are quick, simple and inexpensive. This offers potential to quickly adapt the peptides to the specific conditions, representing a great alternative to existing biofilm and especially biocorrosion protection methods in technical applications. The described methods regarding the covalent immobilization of AMPs onto steel surfaces have been successfully implemented as model projects. Nevertheless, there is still a significant need for ongoing research in order to implement a technical application, especially for covalent attachment in large-scale applications. Similarly, further investigations are necessary, especially for applications in marine environments. Regarding this, parameters such as stability of the peptides at seawater conditions as well as adhesion surface stability should be studied. Regarding the facts still to be tested, transfer of surface coatings with AMP to combat microbiologically induced corrosion represents an attractive and quite promising alternative.
Abbreviations
- AFM:
-
Atomic force microscopy
- AMP:
-
Antimicrobial peptide
- APTES:
-
(3-Aminopropyl)triethoxysilanes
- APTMS:
-
(3-Aminopropyl)trimethoxysilanes
- BPTCS:
-
11-(2-Bromo-2methyl)propionyloxyundecenyltrichlorosilane
- DIEA:
-
N,N-Diisopropylethylamine
- DMF:
-
Dimethylformamide
- EDC:
-
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
- EDX:
-
Energy dispersive x-ray spectroscopy
- EPS:
-
Extracellular polymeric substances
- FT-IR:
-
Fourier-transform infrared spectroscopy
- GPTS:
-
(3-Glycidyloxypropyl)trimethoxysilane
- HBTU:
-
(2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- l-DOPA:
-
L-3,4-Dihydroxyphenylalanin
- MIC:
-
Microbiologically influenced corrosion
- MUA:
-
11-Mercaptoundecanoic acid
- NHS:
-
N-Hydroxysuccinimide
- PEG:
-
Polyethylene glycol
- PGMA:
-
Poly-glycidyl methacrylate
- QCM-D:
-
Quartz crystal microbalance with dissipation monitoring
- SAM:
-
Self-assembly monolayer
- SEM:
-
Scanning electron microscopy
- SOB:
-
Sulphur-oxidizing bacteria
- SPR:
-
Surface plasmon resonance spectroscopy
- SRB:
-
Sulfate-reducing bacteria
- SS:
-
Stainless steel
- TEM:
-
Transmission electron microscopy
- ToF-SIMS:
-
Time-of-flight secondary ion mass spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray diffraction
References
Flemming HC (1994) Microbial deterioration of materials—fundamentals: economical and technical overview. Mikrobielle Werkstoffzerstoerung—Grundlagen: Oekonomisch-technischer Ueberblick. Werkstoffe und Korrosion (Germany) 45:1
Jacobson GA (2007) Corrosion at Purdhoe Bay: a lesson on the line. Mater Perform 46(8):26–32
Dou W, Xu D, Gu T (2021) Biocorrosion caused by microbial biofilms is ubiquitous around us. Microb Biotechnol 14:803–805. https://doi.org/10.1111/1751-7915.13690
Javaherdashti R (1999) A review of some characteristics of MIC caused by sulfate-reducing bacteria: past, present and future. Anti-Corrosion Meth Mater 46:173–180. https://doi.org/10.1108/00035599910273142
Mercer AD (1983) Microbial corrosion: proceedings of the conference sponsored and organized jointly by the National Physical Laboratory and the Metals Society and held at NPL Teddington on 8–10 March 1983. Metals Society, London
Herisson J, van Hullebusch ED, Moletta-Denat M, Taquet P, Chaussadent T (2013) Toward an accelerated biodeterioration test to understand the behavior of Portland and calcium aluminate cementitious materials in sewer networks. Int Biodeterior Biodegrad 84:236–243. https://doi.org/10.1016/j.ibiod.2012.03.007
Singh AK (2020) Industrial cases of microbial induced corrosion. In: Singh AK, D’Silva (eds) Microbially induced corrosion and its mitigation. [S.l.]. Springer Nature, pp 81–106. https://doi.org/10.1007/978-981-15-8019-2_5
Unsal T, Wang D, Kumseranee S, Punpruk S, Gu T (2021) D-Tyrosine enhancement of microbiocide mitigation of carbon steel corrosion by a sulfate reducing bacterium biofilm. World J Microbiol Biotechnol 37:103. https://doi.org/10.1007/s11274-021-03072-9
Zhang P, Xu D, Li Y, Yang K, Gu T (2015) Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 101:14–21. https://doi.org/10.1016/j.bioelechem.2014.06.010
Dai X, Wang H, Ju L-K, Cheng G, Cong H, Newby BZ (2016) Corrosion of aluminum alloy 2024 caused by Aspergillus niger. Int Biodeterior Biodegradation 115:1–10. https://doi.org/10.1016/j.ibiod.2016.07.009
Wang D, Liu J, Jia R, Dou W, Kumseranee S, Punpruk S et al (2020) Distinguishing two different microbiologically influenced corrosion (MIC) mechanisms using an electron mediator and hydrogen evolution detection. Corr Sci 177:108993. https://doi.org/10.1016/j.corsci.2020.108993
Ahmadkhaniha D, Järvenpää A, Jaskari M, Sohi MH, Zarei-Hanzaki A, Fedel M et al (2016) Microstructural modification of pure Mg for improving mechanical and biocorrosion properties. J Mech Behav Biomed Mater 61:360–370. https://doi.org/10.1016/j.jmbbm.2016.04.015
Ilhan-Sungur E, Unsal-Istek T, Cansever N (2015) Microbiologically influenced corrosion of galvanized steel by Desulfovibrio sp. and Desulfosporosinus sp. in the presence of Ag–Cu ions. Mater Chem Phys 162:839–851. https://doi.org/10.1016/j.matchemphys.2015.07.012
Saleem Khan M, Li Z, Yang K, Xu D, Yang C, Liu D et al (2019) Microbiologically influenced corrosion of titanium caused by aerobic marine bacterium Pseudomonas aeruginosa. J Mater Sci Technol 35:216–222. https://doi.org/10.1016/j.jmst.2018.08.001
Jia R, Unsal T, Xu D, Lekbach Y, Gu T (2019) Microbiologically influenced corrosion and current mitigation strategies: a state of the art review. Int Biodeterior Biodegrad 137:42–58. https://doi.org/10.1016/j.ibiod.2018.11.007
Chugh B, Thakur S, Singh AK (2020) Microbiologically influenced corrosion inhibition in oil and gas industry. In: Saji VS, Umoren SA (eds) Corrosion inhibitors in the oil and gas industry. Wiley-VCH, Weinheim, pp 321–338. https://doi.org/10.1002/9783527822140.ch13
Ru Jia DY, Yingchao L, Amir Z, Tingyue G (2017) A novel peptide at a very low concentration enhanced biocide treatment of corrosive biofilms. Paper presented at the CORROSION 2017, New Orleans, Louisiana, USA (Published: March 26 2017)
Wen J, Zhao K, Gu T, Raad II (2009) A green biocide enhancer for the treatment of sulfate-reducing bacteria (SRB) biofilms on carbon steel surfaces using glutaraldehyde. Int Biodeterior Biodegrad 63:1102–1106. https://doi.org/10.1016/j.ibiod.2009.09.007
Wen J, Xu D, Gu T, Raad I (2012) A green triple biocide cocktail consisting of a biocide, EDDS and methanol for the mitigation of planktonic and sessile sulfate-reducing bacteria. World J Microbiol Biotechnol 28:431–435. https://doi.org/10.1007/s11274-011-0832-1
Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L et al (1996) Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol 178:6618–6622. https://doi.org/10.1128/jb.178.22.6618-6622.1996
Scarascia G, Wang T, Hong P-Y (2016) Quorum sensing and the use of quorum quenchers as natural biocides to inhibit sulfate-reducing bacteria. Antibiotics (Basel) 5(4):39. https://doi.org/10.3390/antibiotics5040039
Liu R, Memarzadeh K, Chang B, Zhang Y, Ma Z, Allaker RP et al (2016) Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci Rep 6:29985. https://doi.org/10.1038/srep29985
Gao N, Chen Y, Jiang J (2013) Ag@Fe2O3-GO nanocomposites prepared by a phase transfer method with long-term antibacterial property. ACS Appl Mater Interfaces 5:11307–11314. https://doi.org/10.1021/am403538j
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:D1087–D1093. https://doi.org/10.1093/nar/gkv1278
Di Luca M, Maccari G, Maisetta G, Batoni G (2015) BaAMPs: the database of biofilm-active antimicrobial peptides. Biofouling 31:193–199. https://doi.org/10.1080/08927014.2015.1021340
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. https://doi.org/10.1038/nrmicro1098
Jayaraman A, Mansfeld FB, Wood TK (1999) Inhibiting sulfate-reducing bacteria in biofilms by expressing the antimicrobial peptides indolicidin and bactenecin. J Ind Microbiol Biotechnol 22:167–175. https://doi.org/10.1038/sj.jim.2900627
Trepos R, Cervin G, Pile C, Pavia H, Hellio C, Svenson J (2015) Evaluation of cationic micropeptides derived from the innate immune system as inhibitors of marine biofouling. Biofouling 31:393–403. https://doi.org/10.1080/08927014.2015.1048238
Herzberg M, Berglin M, Eliahu S, Bodin L, Agrenius K, Zlotkin A, Svenson J (2021) Efficient prevention of marine biofilm formation employing a surface-grafted repellent marine peptide. ACS Appl Bio Mater 4:3360–3373. https://doi.org/10.1021/acsabm.0c01672
Kazemzadeh-Narbat M, Cheng H, Chabok R, Alvarez MM, La Fuente-Nunez C de, Phillips KS, Khademhosseini A (2021) Strategies for antimicrobial peptide coatings on medical devices: a review and regulatory science perspective. Crit Rev Biotechnol 41:94–120. https://doi.org/10.1080/07388551.2020.1828810
Andrea A, Molchanova N, Jenssen H (2018) Antibiofilm peptides and peptidomimetics with focus on surface immobilization. Biomolecules 8(2):27. https://doi.org/10.3390/biom8020027
Siedenbiedel F, Tiller JC (2012) Antimicrobial polymers in solution and on surfaces: overview and functional principles. Polymers 4:46–71. https://doi.org/10.3390/polym4010046
Chen J, Shi X, Zhu Y, Chen Y, Gao M, Gao H et al (2020) On-demand storage and release of antimicrobial peptides using Pandora’s box-like nanotubes gated with a bacterial infection-responsive polymer. Theranostics 10:109–122. https://doi.org/10.7150/thno.38388
Zhang T, Wei Q, Zhou H, Zhou W, Fan D, Lin X et al (2020) Sustainable release of vancomycin from micro-arc oxidised 3D-printed porous Ti6Al4V for treating methicillin-resistant Staphylococcus aureus bone infection and enhancing osteogenesis in a rabbit tibia osteomyelitis model. Biomater Sci 8:3106–3115. https://doi.org/10.1039/c9bm01968e
Kelson AB, Carnevali M, Truong-Le V (2013) Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Curr Opin Pharmacol 13:707–716. https://doi.org/10.1016/j.coph.2013.07.001
Shirai T, Shimizu T, Ohtani K, Zen Y, Takaya M, Tsuchiya H (2011) Antibacterial iodine-supported titanium implants. Acta Biomater 7:1928–1933. https://doi.org/10.1016/j.actbio.2010.11.036
Rodríguez López AdL, Lee M-R, Ortiz BJ, Gastfriend BD, Whitehead R, Lynn DM, Palecek SP (2019) Preventing S. aureus biofilm formation on titanium surfaces by the release of antimicrobial β-peptides from polyelectrolyte multilayers. Acta Biomaterialia 93:50–62. https://doi.org/10.1016/j.actbio.2019.02.047
Kord Forooshani P, Lee BP (2017) Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J Polym Sci A Polym Chem 55:9–33. https://doi.org/10.1002/pola.28368
Torres LMFC, Braga NA, Gomes IP, Almeida MT, Santos TL, de Mesquita JP et al (2018) Nanobiostructure of fibrous-like alumina functionalized with an analog of the BP100 peptide: synthesis, characterization and biological applications. Colloids Surf B Biointerfaces 163:275–283. https://doi.org/10.1016/j.colsurfb.2018.01.001
Torres LMFC, Almeida MT, Santos TL, Marinho LES, de Mesquita JP, Da Silva LM et al (2019) Antimicrobial alumina nanobiostructures of disulfide- and triazole-linked peptides: synthesis, characterization, membrane interactions and biological activity. Colloids Surf B Biointerfaces 177:94–104. https://doi.org/10.1016/j.colsurfb.2019.01.052
Wang T, Zou C, Wen N, Liu X, Meng Z, Feng S et al (2021) The effect of structural modification of antimicrobial peptides on their antimicrobial activity, hemolytic activity, and plasma stability. J Pept Sci 27:e3306. https://doi.org/10.1002/psc.3306
Gabriel M, Nazmi K, Veerman EC, Nieuw Amerongen AV, Zentner A (2006) Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjug Chem 17:548–550. https://doi.org/10.1021/bc050091v
Mauchauffé R, Moreno-Couranjou M, Boscher ND, van de Weerdt C, Duwez A-S, Choquet P (2014) Robust bio-inspired antibacterial surfaces based on the covalent binding of peptides on functional atmospheric plasma thin films. J Mater Chem B 2:5168–5177. https://doi.org/10.1039/c4tb00503a
Chen G, Zhou M, Chen S, Lv G, Yao J (2009) Nanolayer biofilm coated on magnetic nanoparticles by using a dielectric barrier discharge glow plasma fluidized bed for immobilizing an antimicrobial peptide. Nanotechnology 20:465706. https://doi.org/10.1088/0957-4484/20/46/465706
Duday D, Vreuls C, Moreno M, Frache G, Boscher ND, Zocchi G et al (2013) Atmospheric pressure plasma modified surfaces for immobilization of antimicrobial nisin peptides. Surf Coat Technol 218:152–161. https://doi.org/10.1016/j.surfcoat.2012.12.045
Diaz-Rodriguez S, Chevallier P, Mantovani D (2018) Low-pressure plasma treatment for direct amination of L605 CoCr alloy for the further covalent grafting of molecules. Plasma Process Polym 15:1700214. https://doi.org/10.1002/ppap.201700214
Godoy-Gallardo M, Mas-Moruno C, Yu K, Manero JM, Gil FJ, Kizhakkedathu JN, Rodriguez D (2015) Antibacterial properties of hLf1-11 peptide onto titanium surfaces: a comparison study between silanization and surface initiated polymerization. Biomacromol 16:483–496. https://doi.org/10.1021/bm501528x
Vreuls C, Zocchi G, Thierry B, Garitte G, Griesser SS, Archambeau C et al (2010) Prevention of bacterial biofilms by covalent immobilization of peptides onto plasma polymer functionalized substrates. J Mater Chem 20:8092. https://doi.org/10.1039/c0jm01419b
Riordan L, Smith EF, Mills S, Hudson J, Stapley S, Nikoi N-D et al (2019) Directly bonding antimicrobial peptide mimics to steel and the real world applications of these materials. Mater Sci Eng C Mater Biol Appl 102:299–304. https://doi.org/10.1016/j.msec.2019.03.064
Šimončicová J, Kryštofová S, Medvecká V, Ďurišová K, Kaliňáková B (2019) Technical applications of plasma treatments: current state and perspectives. Appl Microbiol Biotechnol 103:5117–5129. https://doi.org/10.1007/s00253-019-09877-x
Mas-Moruno C, Garrido B, Rodriguez D, Ruperez E, Gil FJ (2015) Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J Mater Sci Mater Med 26:109. https://doi.org/10.1007/s10856-015-5445-z
Yildirim E, Choi H, Schulte A, Schönherr H (2021) Synthesis of end group-functionalized PGMA-peptide brush platforms for specific cell attachment by interface-mediated dissociative electron transfer reversible addition-fragmentation chain transfer radical (DET-RAFT) polymerization. Eur Polymer J 148:110370. https://doi.org/10.1016/j.eurpolymj.2021.110370
Cao P, Yuan C, Xiao J, He X, Bai X (2018) A biofilm resistance surface yielded by grafting of antimicrobial peptides on stainless steel surface. Surf Interface Anal 50:516–521. https://doi.org/10.1002/sia.6406
Alas GR, Agarwal R, Collard DM, García AJ (2017) Peptide-functionalized polyoligo(ethylene glycol) methacrylate brushes on dopamine-coated stainless steel for controlled cell adhesion. Acta Biomater 59:108–116. https://doi.org/10.1016/j.actbio.2017.06.033
Swan EEL, Popat KC, Desai TA (2005) Peptide-immobilized nanoporous alumina membranes for enhanced osteoblast adhesion. Biomaterials 26:1969–1976. https://doi.org/10.1016/j.biomaterials.2004.07.001
Nie B, Long T, Li H, Wang X, Yue B (2017) A comparative analysis of antibacterial properties and inflammatory responses for the KR-12 peptide on titanium and PEGylated titanium surfaces. RSC Adv 7:34321–34330. https://doi.org/10.1039/C7RA05538B
Khanjanzadeh H, Behrooz R, Bahramifar N, Pinkl S, Gindl-Altmutter W (2019) Application of surface chemical functionalized cellulose nanocrystals to improve the performance of UF adhesives used in wood based composites—MDF type. Carbohyd Polym 206:11–20. https://doi.org/10.1016/j.carbpol.2018.10.115
Majhi S, Peddiraju VC, Mishra A (2021) Effect of antimicrobial peptide (AMP)–tethered stainless steel surfaces on the bacterial membrane. Mater Today Chem 21:100541. https://doi.org/10.1016/j.mtchem.2021.100541
Aditya A, Chattopadhyay S, Gupta N, Alam S, Veedu AP, Pal M et al (2019) ZnO nanoparticles modified with an amphipathic peptide show improved photoprotection in skin. ACS Appl Mater Interfaces 11:56–72. https://doi.org/10.1021/acsami.8b08431
Cao L, Wang L, Fan L, Xiao W, Lin B, Xu Y et al (2017) RGDC peptide-induced biomimetic calcium phosphate coating formed on AZ31 magnesium alloy. Materials (Basel) 10(4):358. https://doi.org/10.3390/ma10040358
Pang LQ, Zhong LJ, Zhou HF, Wu XE, Chen XD (2015) Grafting of ionic liquids on stainless steel surface for antibacterial application. Colloids Surf B Biointerfaces 126:162–168. https://doi.org/10.1016/j.colsurfb.2014.12.018
Bekmurzayeva A, Duncanson WJ, Azevedo HS, Kanayeva D (2018) Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater Sci Eng C Mater Biol Appl 93:1073–1089. https://doi.org/10.1016/j.msec.2018.08.049
Magalhães S, Alves L, Medronho B, Fonseca AC, Romano A, Coelho JFJ, Norgren M (2019) Brief overview on bio-based adhesives and sealants. Polymers 11(10):1685. https://doi.org/10.3390/polym11101685
Rosenthal A, Mantz A, Nguyen A, Bittrich E, Schubert E, Schubert M et al (2018) Biofunctionalization of titanium substrates using nanoscale polymer brushes with cell adhesion peptides. J Phys Chem B 122:6543–6550. https://doi.org/10.1021/acs.jpcb.8b02407
Hofman AH, van Hees IA, Yang J, Kamperman M (2018) Bioinspired underwater adhesives by using the supramolecular toolbox. Adv Mater 30:e1704640. https://doi.org/10.1002/adma.201704640
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430. https://doi.org/10.1126/science.1147241
Jaramillo J, Rodriguez-Oliva I, Abian O, Palomo JM (2020) Specific chemical incorporation of l-DOPA and functionalized l-DOPA-hyaluronic acid in Candida antarctica lipase: creating potential mussel-inspired bioadhesives. SN Appl Sci 2:1–12. https://doi.org/10.1007/s42452-020-03545-w
Yang J, Saggiomo V, Velders AH, Cohen Stuart MA, Kamperman M (2016) Reaction pathways in catechol/primary amine mixtures: a window on crosslinking chemistry. PLoS ONE 11:e0166490. https://doi.org/10.1371/journal.pone.0166490
Cao P, Liu K, Liu X, Sun W, Wu D, Yuan C et al (2020) Antibacterial properties of Magainin II peptide onto 304 stainless steel surfaces: A comparison study of two dopamine modification methods. Colloids Surf B Biointerfaces 194:111198. https://doi.org/10.1016/j.colsurfb.2020.111198
Cao P, Yang Y, Uche FI, Hart SR, Li W-W, Yuan C (2018) Coupling plant-derived cyclotides to metal surfaces: an antibacterial and antibiofilm study. Int J Mol Sci 19(3):793. https://doi.org/10.3390/ijms19030793
Cao P, Li W-W, Morris AR, Horrocks PD, Yuan C-Q, Yang Y (2018) Investigation of the antibiofilm capacity of peptide-modified stainless steel. R Soc Open Sci 5:172165. https://doi.org/10.1098/rsos.172165
Cao P, Du C, He X, Zhang C, Yuan C (2020) Modification of a derived antimicrobial peptide on steel surface for marine bacterial resistance. Appl Surf Sci 510:145512. https://doi.org/10.1016/j.apsusc.2020.145512
Ryu JH, Messersmith PB, Lee H (2018) Polydopamine surface chemistry: a decade of discovery. ACS Appl Mater Interfaces 10:7523–7540. https://doi.org/10.1021/acsami.7b19865
Levin A, Hakala TA, Schnaider L, Bernardes GJL, Gazit E, Knowles TPJ (2020) Biomimetic peptide self-assembly for functional materials. Nat Rev Chem 4:615–634. https://doi.org/10.1038/s41570-020-0215-y
Ulman A (1996) Formation and structure of self-assembled monolayers. Chem Rev 96:1533–1554. https://doi.org/10.1021/cr9502357
Zhang Z, Kou N, Ye W, Wang S, Lu J, Lu Y et al (2021) Construction and characterizations of antibacterial surfaces based on self-assembled monolayer of antimicrobial peptides (Pac-525) derivatives on gold. Coatings 11(9):1014. https://doi.org/10.3390/coatings11091014
Humblot V, Yala J-F, Thebault P, Boukerma K, Héquet A, Berjeaud J-M, Pradier C-M (2009) The antibacterial activity of Magainin I immobilized onto mixed thiols self-assembled monolayers. Biomaterials 30:3503–3512. https://doi.org/10.1016/j.biomaterials.2009.03.025
Brown MA, Cortes-Lobos R, Cox M (2011) Chapter 12—reinventing industrial energy use in a resource-constrained world. In: Sioshansi FP (ed) Energy, sustainability and the environment. Butterworth-Heinemann, Boston, pp 337–366. https://doi.org/10.1016/B978-0-12-385136-9.10012-9
Sudha E, Selvam R, Sivaswaroop P, Chandran KPS (2017) Diimide activated coupling of carbon nanotubes with chemically addressable peptide template for biomedical applications. Indian J Chem Technol 67–72
Wilke G (1979) Organo transition metal compounds as intermediates in homogeneous catalytic reactions. In: Ishii Y, Hagihara N (eds) Organometallic chemistry: pergamon, pp 677–690. https://doi.org/10.1016/B978-0-08-022035-2.50012-8
Takakura A, Beppu K, Nishihara T, Fukui A, Kozeki T, Namazu T et al (2019) Strength of carbon nanotubes depends on their chemical structures. Nat Commun 10:3040. https://doi.org/10.1038/s41467-019-10959-7
Poh CK, Shi Z, Tan XW, Liang ZC, Foo XM, Tan HC et al (2011) Cobalt chromium alloy with immobilized BMP peptide for enhanced bone growth. J Orthop Res 29:1424–1430. https://doi.org/10.1002/jor.21409
Tan HC, Poh CK, Cai Y, Wang W (2013) Anti-fibrosis effect of BMP-7 peptide functionalization on cobalt chromium alloy. J Orthop Res 31:983–990. https://doi.org/10.1002/jor.22313
Campbell D, Carnell SM, Eden RJ (2013) Applicability of contact angle techniques used in the analysis of contact lenses, part 1: comparative methodologies. Eye Contact Lens 39:254–262. https://doi.org/10.1097/ICL.0b013e31828ca174
Puiu M, Bala C (2016) SPR and SPR imaging: recent trends in developing nanodevices for detection and real-time monitoring of biomolecular events. Sensors (Basel) 16(6):870. https://doi.org/10.3390/s16060870
Nabok AV, Tsargorodskaya A, Hassan AK, Starodub NF (2005) Total internal reflection ellipsometry and SPR detection of low molecular weight environmental toxins. Appl Surf Sci 246:381–386. https://doi.org/10.1016/j.apsusc.2004.11.084
Morent R, de Geyter N, Leys C, Gengembre L, Payen E (2008) Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf Interface Anal 40:597–600. https://doi.org/10.1002/sia.2619
Andrade JD (1985) X-ray photoelectron spectroscopy (XPS). In: Andrade JD (ed) Surface and interfacial aspects of biomedical polymers. Springer US, Boston, MA, pp 105–195. https://doi.org/10.1007/978-1-4684-8610-0_5
Zhang X-F, Liu Z-G, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17(9):1534. https://doi.org/10.3390/ijms17091534
Klemm D, Stangl M, Peeva A, Hoffmann V, Wetzig K, Eckert J (2008) Analysis of interface impurities in electroplated Cu layers by using GD-OES and TOF-SIMS. Surf Interface Anal 40:418–422. https://doi.org/10.1002/sia.2743
Arenas-Alatorre J, Silva-Velazquez Y, Alva Medina A, Rivera M (2010) Advantages and limitations of OM, SEM, TEM and AFM in the study of ancient decorated pottery. Appl Phys A 98:617–624. https://doi.org/10.1007/s00339-009-5451-4
Glover RD, Miller JM, Hutchison JE (2011) Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano 5:8950–8957. https://doi.org/10.1021/nn2031319
Dutta D, Kumar N, Willcox MDP (2016) Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling 32:429–38. https://doi.org/10.1080/08927014.2015.1129533
Kang S-J, Won H-S, Choi W-S, Lee B-J (2009) De novo generation of antimicrobial LK peptides with a single tryptophan at the critical amphipathic interface. J Pept Sci 15:583–588. https://doi.org/10.1002/psc.1149
Béven L, Castano S, Dufourcq J, Wieslander A, Wróblewski H (2003) The antibiotic activity of cationic linear amphipathic peptides: lessons from the action of leucine/lysine copolymers on bacteria of the class Mollicutes. Eur J Biochem 270:2207–2217. https://doi.org/10.1046/j.1432-1033.2003.03587.x
Carmona G, Rodriguez A, Juarez D, Corzo G, Villegas E (2013) Improved protease stability of the antimicrobial peptide Pin2 substituted with D-amino acids. Protein J 32:456–466. https://doi.org/10.1007/s10930-013-9505-2
Lu J, Xu H, Xia J, Ma J, Xu J, Li Y, Feng J (2020) D- and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Front Microbiol 11:563030. https://doi.org/10.3389/fmicb.2020.563030
Haney EF, Barbosa SC, Baquir B, Hancock REW (2019) Influence of non-natural cationic amino acids on the biological activity profile of innate defense regulator peptides. J Med Chem 62:10294–10304. https://doi.org/10.1021/acs.jmedchem.9b01344
Shao C, Zhu Y, Lai Z, Tan P, Shan A (2019) Antimicrobial peptides with protease stability: progress and perspective. Future Med Chem 11:2047–2050. https://doi.org/10.4155/fmc-2019-0167
Avan I, Hall CD, Katritzky AR (2014) Peptidomimetics via modifications of amino acids and peptide bonds. Chem Soc Rev 43:3575–3594. https://doi.org/10.1039/C3CS60384A
Jia R, Yang D, Dou W, Liu J, Zlotkin A, Kumseranee S et al (2019) A sea anemone-inspired small synthetic peptide at sub-ppm concentrations enhanced biofilm mitigation. Int Biodeterior Biodegrad 139:78–85. https://doi.org/10.1016/j.ibiod.2018.11.009
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stillger, L., Müller, D. Peptide-coating combating antimicrobial contaminations: a review of covalent immobilization strategies for industrial applications. J Mater Sci 57, 10863–10885 (2022). https://doi.org/10.1007/s10853-022-07266-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07266-w